The prognostic impact of body mass index in breast cancer according to tumor subtype
Article information
Abstract
Purpose
Several studies demonstrated that obesity and underweight were negatively associated with outcomes of breast cancer. However, the results are still controversial, and the impact of body mass index (BMI) on distant metastasis-free survival (MFS), which might directly affect mortality, was less well evaluated. Our study aimed to verify the prognostic effect of BMI in breast cancer.
Methods
A retrospective analysis of 504 patients with stage I–III breast cancer who underwent surgery from January 2005 to December 2013 was performed. The patients were divided into three groups according to preoperative BMI: underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, and overweight ≥25 kg/m2. The association between body weight status and breast cancer recurrence was analyzed. Subgroup analysis by tumor subtype according to receptor status was also performed.
Results
The median follow-up period was 88 months. For disease recurrence, histologic grade and human epidermal growth factor receptor 2 (HER2)-positivity were independent prognostic factors in multivariate analysis. Stage, histologic grade, HER2-positivity, and BMI status were independent prognostic factors for distant metastasis. In survival analysis, overweight and underweight were significant predisposing factors for MFS, but not for disease-free survival (DFS). In the estrogen receptor (ER)-positive group, overweight and underweight patients had significantly worse DFS and MFS than normal weight patients. In the ER-negative or HER2-positive group, BMI status had no significant association with DFS and MFS.
Conclusion
The prognostic role of BMI on the survival outcomes of patients with breast cancer was different by tumor subtype. In ER-positive patients, overweight and underweight statuses had a negative prognostic effect on DFS and MFS, respectively.
INTRODUCTION
Breast cancer is the most common cancer in women worldwide and the second most common cause of cancer-related deaths in women [1]. The incidence of breast cancer is increasing annually, and the prevalence of obesity has also rapidly increased in South Korea [2]. Many studies investigated the prognostic impact of body mass index (BMI) on breast cancer, but the results are controversial. Some of the studies reported that body weight status was significantly associated with breast cancer prognosis [3–5], while other studies did not identify the association [6,7]. Most previous studies investigated the prognostic effect of BMI by conducting analyses of overall survival. However, a recent nationwide study reported that cancer deaths had decreased, and the risk of non-cancer deaths, including underlying diseases especially due to heart disease, septicemia, and self-inflicted injury, had increased in breast cancer patients [8]. The factor contributing to cancer-related mortality is often attributed to the extensive metastatic dissemination of the disease throughout the body and result in organ dysfunction. Therefore, to verify the prognostic impact of BMI on breast cancer, direct influence of BMI to distant metastasis-free survival (MFS) is important. However, previous studies did not perform analysis regarding MFS. Our study aimed to investigate the influence of BMI on disease recurrence and distant metastasis, thereby contributing to breast cancer prognosis.
METHODS
Study population
We performed a retrospective cohort study with patients with breast cancer who underwent curative surgery at a single institution from January 2005 to December 2013. All patients were recommended to undergo standard adjuvant treatment after surgery, and regular postoperative surveillance was performed according to the oncologist’s practice. All patients were recommended to complete clinical examinations every 3 to 6 months during the first 2 years after surgery, thereafter every 6 months during 5 years. The exclusion criteria were as follows: patients who had other malignancies, patients with de novo metastasis or in situ disease, and patients who had incomplete data.
Data collection
Body weight and height were measured on the day of admission for breast cancer surgery. BMI was calculated by dividing weight in kilograms by the square of height in meters. BMI was categorized into three groups: underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, and overweight ≥25 kg/m2. We reviewed the electronic medical record, and the clinicopathologic characteristics and clinical outcomes of the enrolled patients regarding disease recurrence and distant metastasis were investigated. Disease-free survival (DFS) was defined as the length of time from surgery to the date of recurrence at any site. MFS was defined as the length of time from surgery to the date of cancer spread to other parts of the body. Subgroups were categorized according to estrogen receptor (ER) status, and human epidermal growth factor receptor 2 (HER2) receptor status. This study protocol was reviewed and approved by the Institutional Review Board of Gyeongsang National University Hospital (IRB No. GNUH 2022-12-034). The requirement for informed consent was waived based on the retrospective design.
Statistical analysis
Continuous variables were presented as mean ± standard deviation. Categorical variables were presented as number with proportion (%). Survival outcomes were estimated by the Kaplan-Meier method, and the log-rank test was used for comparisons across groups. Hazard ratios were calculated by the Cox proportional hazards model. All statistical analyses were performed with SPSS (version 21.0; SPSS, Inc.), and P<0.05 was considered statistically significant.
RESULTS
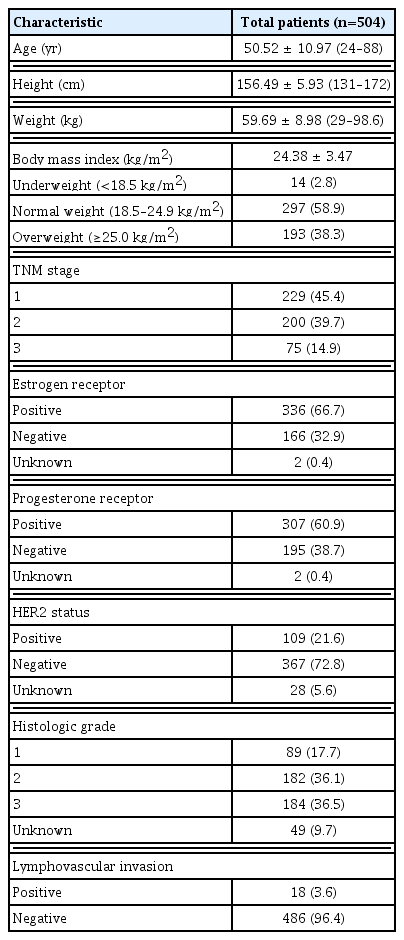
We reviewed the data of 615 patients who underwent surgery in our institution. Eleven patients had no data of height or weight, 35 patients had other malignancies, and 65 patients had in situ disease or de novo metastasis. A total of 504 patients were included in this study. The clinicopathologic characteristics of the patients are presented in Table 1. The median follow-up period was 88 months (range, 6–134 months). The tumor stage was I in 229 (45.4%), II in 200 (39.7%), and III in 75 (14.9%) patients. Seventy-one patients (13.8%) experienced disease recurrence, and 56 patients (10.9%) experienced distant metastasis. The mean BMI value was 24.38 kg/m2. According to the BMI categorizations, 14 patients (2.8%) were underweight, 297 patients (58.9%) were normal weight, and 193 patients (38.3%) were overweight.
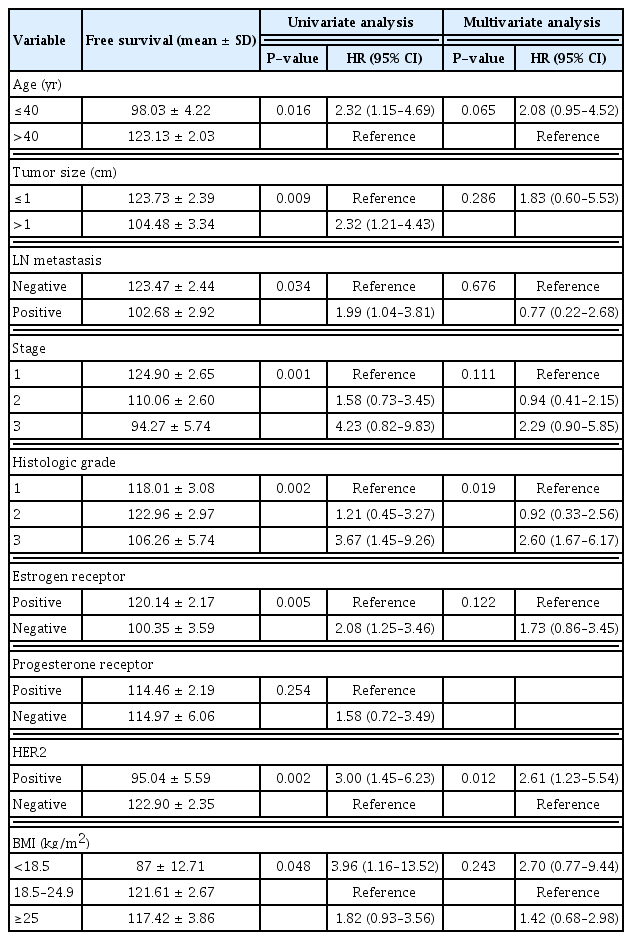
To verify the prognostic role of characteristics on disease recurrence, we performed univariate and multivariate analyses. As seen in Table 2, age at diagnosis, tumor size, the presence of lymph node metastasis, tumor stage, histologic grade, ER status, HER2 status, and BMI were significantly associated with disease recurrence in univariate analysis. In multivariate analysis, histologic grade and HER2 status were independent risk factors for disease recurrence.
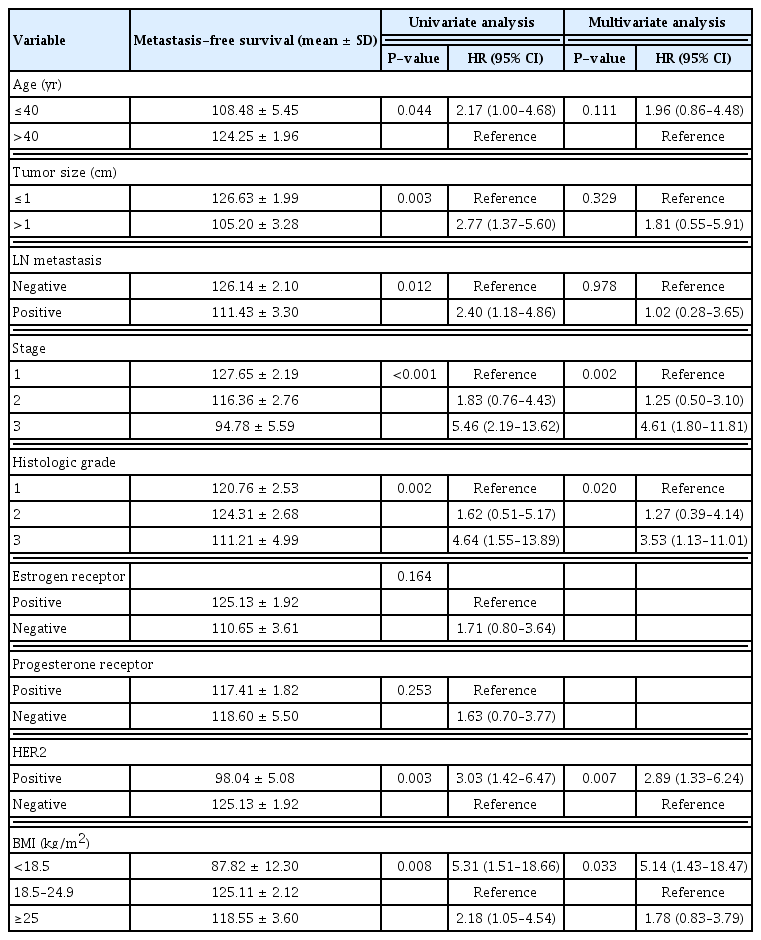
We also analyzed which factors affected distant metastasis. In univariate analysis, age at diagnosis, tumor size, the presence of lymph node metastasis, stage, histologic grade, HER2 status, and BMI were associated with distant metastasis. In multivariate analysis, tumor stage, histologic grade, HER2 status, and BMI showed prognostic significance. Analyses of distant metastasis are presented in Table 3.
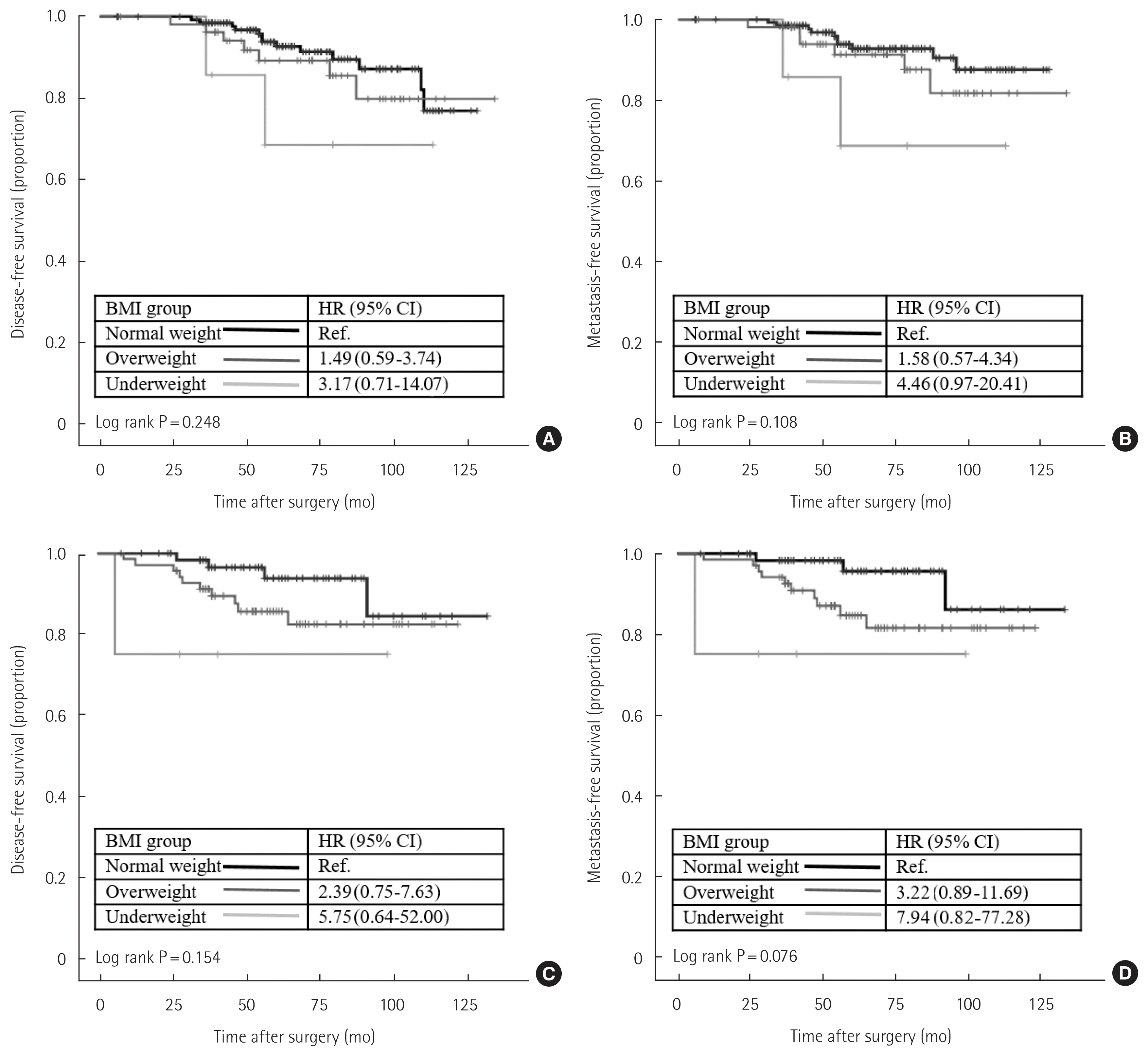
We examined the prognostic effect of BMI on survival by the Kaplan-Meier method. When all study patients were included, BMI showed no significant association with DFS. However, normal weight patients showed better survival outcomes than overweight or underweight patients regarding distant metastasis (Fig. 1A and B). We next performed subgroup analysis by tumor subtype according to ER status and HER2 receptor status. In the ER-positive group, overweight and underweight patients had significantly worse DFS and MFS than normal weight patients (Fig. 1C and D). However, there was no significant difference in DFS and MFS according to BMI status in the ER-negative or HER2-positive patients. We also performed an analysis by incorporating menstruation status to comprehensively verify the prognostic effect of BMI in the ER-positive group. However, menstruation status did not affect DFS or MFS in the ER-positive group (Fig. 2).
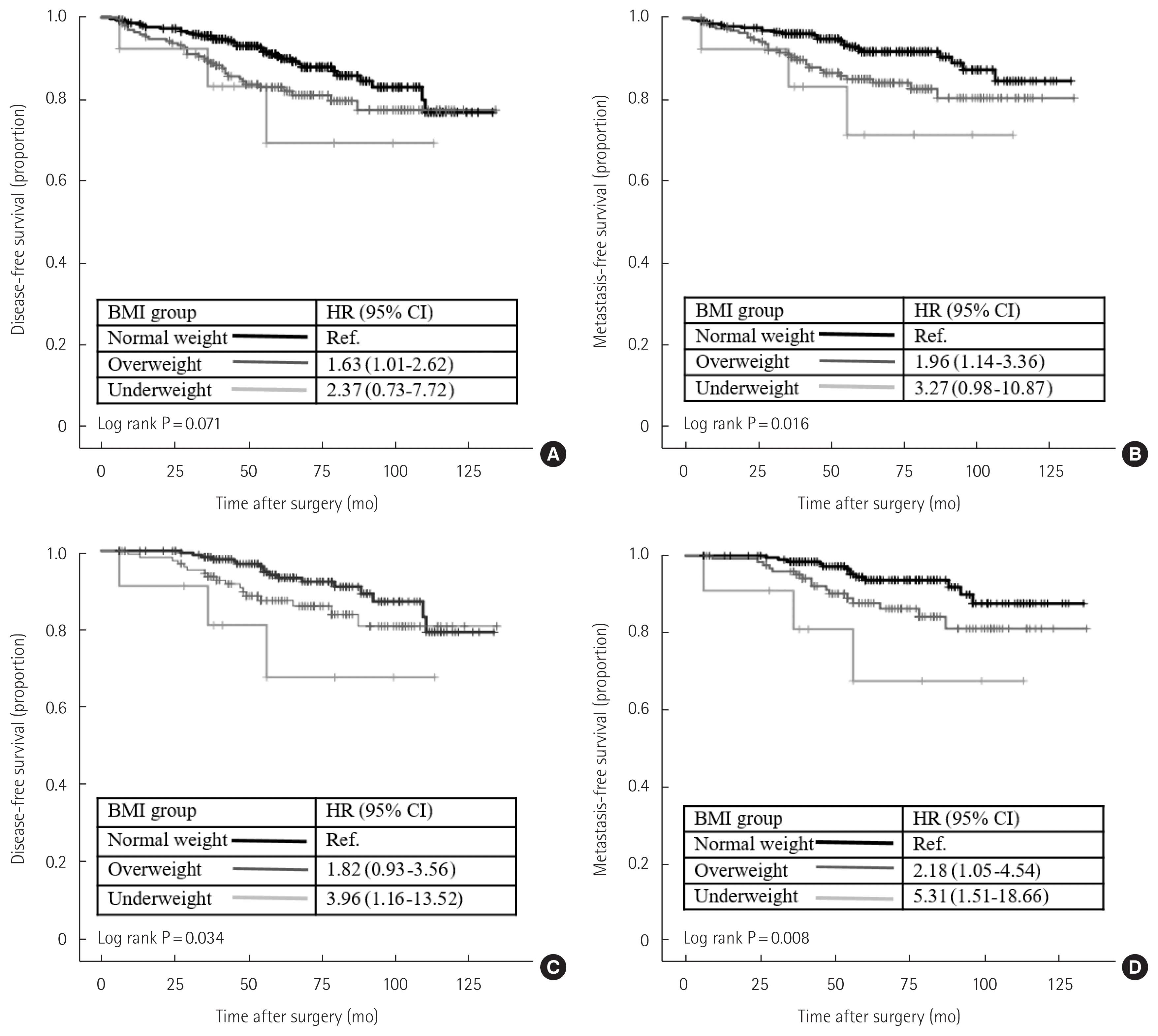
The effect of BMI on breast cancer survival. Kaplan-Meier curves of (A) disease-free survival of all study patients; (B) metastasis-free survival of all study patients; (C) disease-free survival of ER-positive patients; or (D) metastasis-free survival of ER-positive patients. BMI, body mass index; ER, estrogen receptor; HR, hazard ratio; CI, confidence interval. BMI groups were classified as follows: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (≥25.0 kg/m2).
The effect or body mass index on survival according to menstruation status in ER-positive group. Kaplan-Meier curves of (A) disease-free survival of premenopausal patients; (B) metastasis-free survival of premenopausal patients; (C) disease-free survival of postmenopausal patients; or (D) metastasis-free survival of postmenopausal patients. BMI groups were classified as follows: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (≥25.0 kg/m2).
DISCUSSION
In the present study, we demonstrated that overweight and underweight patients had significantly worse prognoses with regard to distant metastasis. In particular, obesity and underweight statuses were significantly associated with breast cancer outcomes in ER-positive patients. In ER-positive patients, obesity and underweight statuses had negative prognostic effects on disease recurrence and distant metastasis.
Previous studies investigated the relationship between BMI and the risk of breast cancer. Many studies reported that obesity was related to the occurrence and prognosis of breast cancer [9–11]. The current consensus in breast cancer research is that obesity is linked to breast cancer development and recurrence in postmenopausal women because endogenous estrogens in postmenopausal women are generated by adipose tissue conversion [12]. A large single-center-based cohort study by Seoul National University Hospital demonstrated that obesity had a negative prognostic impact on relapse-free and overall survival in hormone receptor-positive, HER2-negative postmenopausal patients [13]. Sun et al. [14] reported that obesity and overweight statuses were associated with worse prognoses in postmenopausal patients with breast cancer. In contrast, other studies demonstrated that obesity had a worse prognostic impact on premenopausal women. Berclaz et al. [3] reported that obesity or overweight status was associated with a poor prognosis after breast cancer treatment, especially in premenopausal and perimenopausal patients. Kawai et al. [15] also reported that obesity was significantly associated with mortality in premenopausal patients. Moreover, several meta-analyses of published data showed that obesity was associated with worse overall survival in both pre- and postmenopausal patients with breast cancer [4,16]. Likewise, in our study, obese patients had worse DFS and MFS than normal weight patients, and menstruation status did not alter the impact of obesity on breast cancer outcomes. The mechanism underlying an association between obesity and breast cancer survival has not been well-established, but might be explained by higher levels of insulin, insulin-like growth factor, adipocytokines, and inflammatory cytokines that are linked to cell cycle regulation or apoptosis, and the increased synthesis of estrogen in obese patients with postmenopausal status [16–21]. Treatment-related factors, such as chemotherapy underdosing or the decreased efficacy of endocrine therapy in obese patients, also might have an effect to worsen the survival of breast cancer patients [22,23].
Several studies showed the negative prognostic effect of underweight status in patients with breast cancer. Moon et al. [24] performed analyses of nationwide data and further explored a large single-institution database of 4,345 patients and reported that underweight patients had significantly lower overall survival and breast cancer-specific survival than normal weight patients. The study also showed that underweight women had a significantly higher risk of both distant metastasis and the local recurrence of breast cancer. Xiao et al. [25] reported that the breast cancer death rate for underweight patients was 2.1 times that of normal weight patients. Regarding metastasis breast cancer, Saleh et al. [26] reported that being underweight was an adverse independent prognostic factor for overall survival and progression-free survival. An association between low BMI and poorer prognosis has also been demonstrated in other malignancies, including gastric cancer or colon cancer [27,28]. Several possible mechanisms underlie the association between being underweight and breast cancer survival. Tumor cells and immune cells might have systemic and local interactions. Immune cells may inhibit or promote tumor progression and influence the efficacy of systemic antitumor treatments. In underweight status caused by undernutrition, cytokine reactions and activation of the immune system are compromised, which might affect tumor-immune system interactions [29]. A protective role of mammary adipocytes has been proposed [30] and being underweight, which causes decreases in fat components, might also have an effect on worsening breast cancer outcomes.
There were several limitations to our study. First, we performed a retrospective study, and the prognostic effects must be verified through prospective studies. Second, the number of enrolled patients and recurrence events were quite small, and the follow-up period was relatively short. In particular, underweight patients accounted for only 2.8%, and this population might have bias. Third, the BMI status was checked in the preoperative period. We did not monitor dynamic changes in body weight status, which would happen in the postoperative period. Fourth, we categorized patient BMI status into only three groups, due to relative small number of enrolled patients. According to the WHO classification of weight status, patients are categorized into four groups, including underweight, normal range, overweight, and obese groups. This categorization might have affected the prognostic effect of BMI in patients with high BMI. Moreover, all patients in this cohort were Asian, so we used a cutoff value proposed for an Asian population. Validation with different ethnicities is also needed.
In this study, we found that the prognostic role of BMI on the survival outcomes of patients with breast cancer was different by tumor subtype. In ER-positive patients, overweight and underweight statuses had a negative prognostic effect on disease recurrence and distant metastasis, respectively. Further studies with a large cohort and validation with different ethnicities are required to evaluate the prognostic effect of body weight status on breast cancer outcomes.
Notes
No potential conflict of interest relevant to this article was reported.
Funding
None.