Factors related to compliance with adjuvant chemotherapy in patients with gastric cancer: A retrospective single-center study
Article information
Abstract
Purpose
Adjuvant chemotherapy (AC) improves survival outcomes in patients with advanced gastric cancer (GC) after curative surgery; however, some patients do not receive or complete chemotherapy. This study aimed to identify factors related to patient compliance with chemotherapy after curative surgery for advanced GC.
Methods
The data of patients who underwent curative gastrectomy for pathologic stage II–III GC between 2012 and 2016 were reviewed. Patients were divided into an AC completion group (group C), AC incompletion group (group I), and surgery-only group (group S). The AC regimen was either tegafur/gimeracil/oteracil (S-1) or capecitabine plus oxaliplatin (XELOX).
Results
The study enrolled 417 patients; group C had 222 patients, group I had 110, and group S had 85. The most common reason for not initiating AC was poor general condition (36.5%), while chemotherapy-related complications was the common reason for AC incompletion (43.6%). In multivariate analysis, age over 65 years, Eastern Cooperative Oncology Group performance status ≥1, Charlson comorbidity index ≥1, and the presence of postoperative complications were independent risk factors for not initiating AC (odds ratio: 4.32, 2.62, 1.84, and 2.17, respectively). Age over 65 years, longer postoperative stay, and XELOX regimen were significant risk factors for incompletion of AC (odds ratio: 2.68, 1.72, and 2.23, respectively).
Conclusion
Old age, poor performance status, comorbidities, and postoperative complications, longer postoperative hospital stay, and XELOX regimen were associated with poor compliance with AC in GC patients. Clinicians can improve compliance with AC by managing postoperative complications and selecting the most appropriate treatment regimen.
INTRODUCTION
Radical gastrectomy is the standard treatment for resectable gastric cancer (GC). South Korean and Japanese guidelines recommend adjuvant chemotherapy (AC) after D2 lymph node dissection for patients with pathologic stage II–III to improve survival outcomes [1,2]. In 2007, a randomized clinical trial of tegafur/gimeracil/oteracil (S-1) therapy, the ACTS-GC trial, demonstrated a survival benefit of adjuvant oral fluoropyrimidine therapy compared with surgery alone [3]. Similarly, the CLASSIC trial found that AC with capecitabine plus oxaliplatin (XELOX) after gastrectomy with D2 lymph node dissection should be considered for stage II–III patients [4].
To maximize the benefits of chemotherapy, appropriate treatment schedules and good patient compliance are important. Most studies on the efficacy of XELOX and S-1 were based on eight cycles of AC. Recent studies have shown that starting AC within 8 weeks of surgery, and continuing treatment for more than 6 months, improves survival rates [5,6]. Other studies reported that the completion rate for eight cycles of S-1 or XELOX is approximately 65%–75% [7,8]. Therefore, 25%–35% of patients do not complete or initiate AC for various reasons, including poor physical condition before or after gastrectomy.
Given the current paucity of data, this study aimed to identify factors associated with compliance with AC after gastrectomy for GC.
METHODS
Patients
This single-center cohort study included 440 patients diagnosed with pathologic stage II–III GC, who underwent curative radical gastrectomy at Seoul St. Mary’s Hospital from 2012 to 2016. The exclusion criteria were as follows: previously received neoadjuvant chemotherapy, diagnosed with cancer in another organ within 5 years prior to gastrectomy, and missing data due to death or loss to follow-up. Ultimately, 417 patients were evaluated retrospectively.
Study protocol
Eligible patients were divided into three groups: 222 patients received eight cycles of AC (completion group, group C), while in 110 patients AC was initiated but they ultimately received less than eight cycles of chemotherapy (incompletion group, group I). Finally, 85 patients only underwent gastrectomy without AC (surgery-only group, group S). Baseline characteristics (at the time of gastrectomy) and operative outcomes were extracted from the electric medical records. Comorbidities were quantified using the Charlson comorbidity index (CCI) [9]. The physical status of patients was categorized using the Eastern Cooperative Oncology Group performance status (ECOG-PS). The severity of postoperative complications was classified according to the Clavien-Dindo classification (CDC) [10]. CDC grade ≥3 was taken to indicate severe complications. Depth of invasion and lymph node metastasis were categorized according to the 8th American Joint Committee on Cancer TNM classification. The reasons for discontinuing chemotherapy and not initiating chemotherapy were investigated in groups I and S, respectively. After the AC treatment, patients were followed up regularly according to the standard protocols of our institution. Patients visited the outpatient clinic every 3 months for the first 2 years, and every 6 months thereafter. The median follow-up period was 42 months. This study was approved by the Ethics Committee and Institutional Review Board of Seoul St. Mary’s Hospital (approval No. KC20RASI0937). The informed consent was waived.
Chemotherapy protocols
AC was planned for patients diagnosed with stage II–III GC after curative surgery. According to the policy of our institute, S-1 therapy was mainly used for stage II patients, and XELOX therapy for stage III patients. The treatment generally began about 4 weeks after surgery. The chemotherapy protocols of our institution are as follows. The S-1 regimen consists of eight 4-week cycles of 80–120 mg oral S-1 per square meter of body surface area per day, followed by a “rest period” of 2 weeks. The XELOX regimen consists of eight 3-week cycles of oral capecitabine (1,000 mg/m2 twice daily on days 1–14 of each cycle) plus intravenous oxaliplatin (130 mg/m2 on day 1 of each cycle), followed by a rest period of 1 week. Side effects were assessed based on blood tests performed at the time of initiation of each cycle and 1 week thereafter. Cancer progression was checked using computed tomography and tumor marker tests performed after every three cycles of chemotherapy.
Statistical analysis
The data were analyzed using SPSS for Windows software version 24.0 (IBM Corp., Armonk, NY, USA). Continuous variables were analyzed using Student t-test or one-way analysis of variance. Categorical variables were compared with the chi-square test or Fisher exact test. The Bonferroni post hoc test was also applied. Overall survival (OS) was calculated using the Kaplan–Meier method, and compared among groups using the log-rank test. Factors that differed significantly among groups were subjected to multivariate analysis, with logistic regression used to determine risk factors for chemotherapy compliance and calculate the odds ratios (ORs). P-value <0.05 was considered statistically significant, and P <0.017 was considered significant in the post hoc analysis.
RESULTS
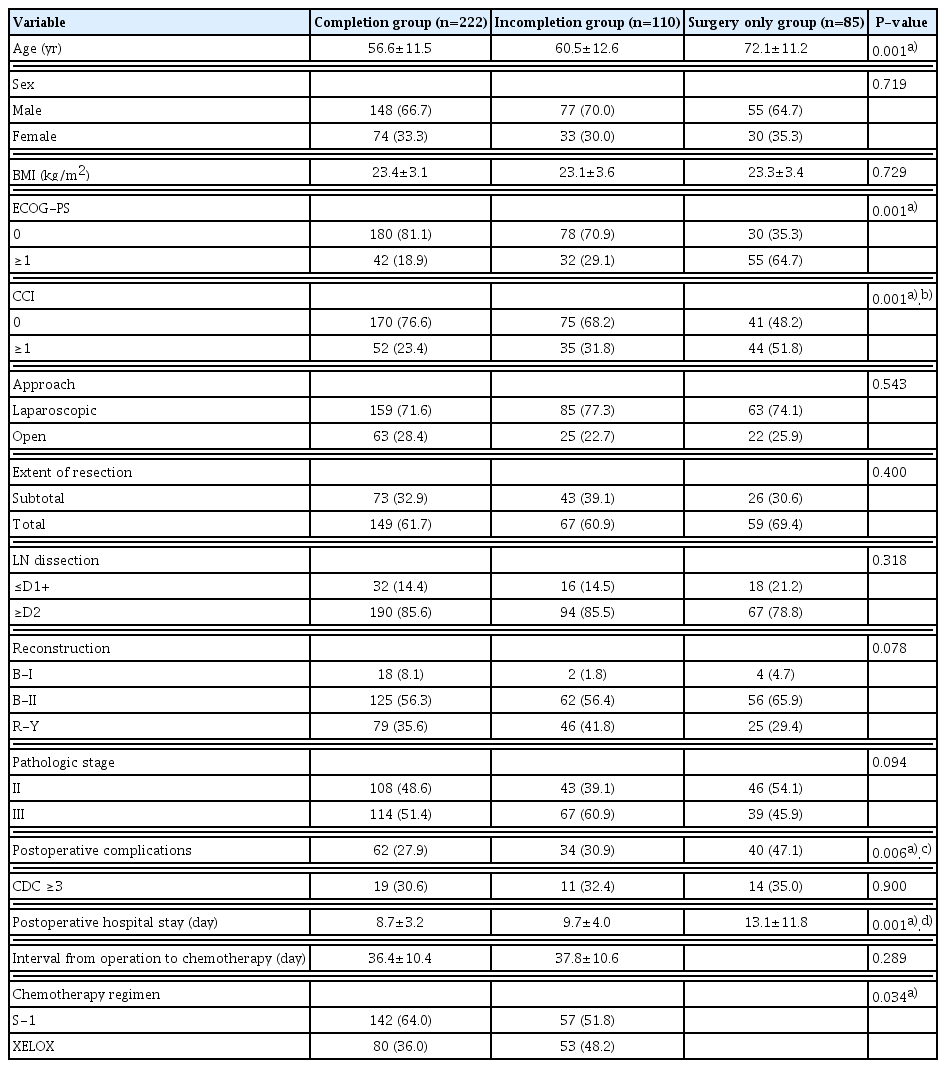
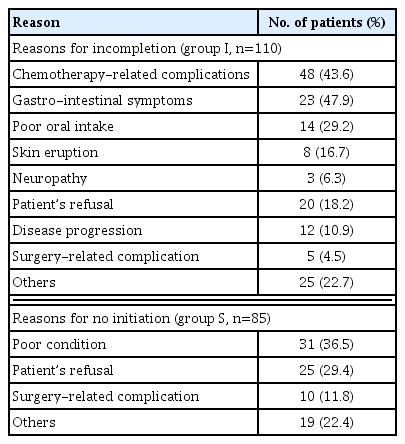
The median follow-up duration was 42 months (range, 1–92 months). For groups C, I and S, the 5-year OS rate was 85.5%, 80.6%, and 64.6%, respectively (P<0.001) (Supplementary Fig. S1). The baseline characteristics and operative details of the three groups are shown in Table 1. The mean age of groups C, I, and S was 56.6, 60.5, and 72.1 years, respectively; group S patients were significantly older than those on the other groups (P<0.001). The ECOG-PS and CCI were significantly different among the three groups, and group S had the highest proportions of patients with an ECOG-PS or CCI ≥1 (64.7% and 51.8%, respectively). The mean postoperative length of stay (PLOS) was significantly longer in group S than groups C and I (13.1, 8.7 and 9.7 days, respectively, P<0.001). The proportion of patients receiving S-1 was significantly higher in group C than group I (64.0% and 51.8%, respectively, P=0.034). The most common reasons for not receiving chemotherapy were poor general condition (36.5%) and patient refusal (29.4%), while chemotherapy-related complications was the major reason for incompletion thereof (43.6%) (Table 2).
An age over 65 years, PLOS over 7 days, and use of XELOX were significant risk factors for incompletion of AC (OR: 2.68, 1.72, and 2.23, respectively) (Table 3). An age over 65 years, ECOG-PS ≥1, CCI ≥1, and presence of postoperative complications were significant risk factors for not initiating AC (OR: 4.32, 2.62, 1.84, and 2.17, respectively) (Table 4).
DISCUSSION
The benefits of AC for advanced GC have been proven in large-scale, randomized controlled trials. However, some patients do not receive chemotherapy for various reasons, resulting in poor survival outcomes. This study investigated factors associated with compliance with AC. In multivariate analysis, old age, comorbidities, and postoperative complications were risk factors for not initiating AC. Old age, long PLOS, and XELOX regimen were risk factors for discontinuing AC. Clinicians should pay close attention to patients with such risk factors and try to prevent those that are controllable, such as postoperative complications and PLOS.
In this study, old age and comorbidities were risk factors for not initiating chemotherapy (Supplementary Tables S1, S2). One of the most common sequelae after GC surgery is dietary problems, which can lead to weight loss and sarcopenia [11]. A previous study reported that postoperative weight loss can affect compliance with AC in GC patients [12]. Older patients with comorbidities are more likely to experience postoperative dietary problems or severe weight loss, which may reduce compliance with chemotherapy. A previous study reported that old age (>60 years), low body mass index (<23 kg/m2), and poor physical status were associated with lower compliance [13]. Other studies on the S-1 regimen for GC patients reported than an age over 65 years was a significant factor for poor compliance with AC [14,15].
Prolonged PLOS was a risk factor for incompletion of AC in this study. Gastrectomy patients in our institution are discharged on postoperative day 7 following application of the clinical protocol. Therefore, a PLOS over 7 days indicates problems such as operative complications or delayed recovery. Although the proportion of patients with a CDC grade≥3 did not differ significantly among the three groups, that was not the case for CDC grade≥1 patients. This suggests that minor complications, such as gastric stasis and temporary ileus, can also affect postoperative recovery and the patient’s condition. As postoperative complication was a risk factor for incompletion of AC, clinicians should pay close attention to minor complications and complaints after surgery to enhance compliance.
The XELOX was applied in stage III GC patients and S-1 was applied in stage II GC patients at our institution, respectively. A previous study reported that neither of these two regimens was significantly superior to the other [16]. However, other studies have reported that the XELOX regimen may be more effective in stage IIIB and IIIC GC patients, supporting the approach of our institution [7]. In this study, patients on the XELOX regimen were more likely to fail to complete chemotherapy than those on the S-1 regimen. The rate of dose reduction was higher in the XELOX than S-1 group (15% vs. 5.5%, P=0.004) (Supplementary Table S3), suggesting that the XELOX regimen was generally more difficult for patients to maintain. In the CLASSIC trial, grade 3–4 chemotherapy-related adverse events were reported in 56% of the XELOX group, compared to 22.8% of the S-1 group in the ACTS-GC trial. Jang et al. [14] reported a more than two-fold higher rate of grade 3–4 chemotherapy-related adverse events in their XELOX group than the S-1 group (47% vs. 21%) [17]. Finally, oncologic patients tend to prefer oral over intravenous drugs for reasons of convenience, perceived efficacy, and past experience [18]. Therefore, clinicians need to pay more attention to patients on the XELOX regimen.
Thirty-three patients stopped chemotherapy in the first cycle, which was the highest drop-out rate in group I (30%) (Supplementary Fig. S2). Almost two-thirds of patients failed to complete more than half of the entire chemotherapy cycles (70%, n=77). This suggest that patients may be more vulnerable to treatment in the early period. Therefore, it is necessary to focus more on patients with risk factors at the beginning of treatment.
This study had some limitations. First, it used as a retrospective, single center design, which could give rise to certain biases. Some factors found to be significant in other studies, including postoperative weight change, serum creatinine, and sarcopenia, were not analyzed in our study [19]. Second, the results of this study may not generalize to Western countries. While AC is frequently used in Asian countries, perioperative chemotherapy and adjuvant chemoradiation are used more often in Western countries [20,21]. Further research is also needed on the associations of health insurance and socioeconomic status with treatment outcomes.
In conclusion, an age over 65 years, prolonged PLOS, comorbidities, postoperative complications and XELOX treatment were associated with poorer compliance with AC in advanced GC patients. Compliance can be improved by treating postoperative complications appropriately, reducing the postoperative hospital stay, and selecting the appropriate treatment regimen according to the patient’s life expectancy and systemic conditions.
Supplementary Information
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIALS
Supplementary materials are available at the Korean Journal of Clinical Oncology website (http://www.kjco.org/).