The outcomes of intensified 5-fluorouracil plus leucovorin chemotherapy for preoperative chemoradiation in rectal cancers
Article information
Abstract
Purpose
The clinical benefit of intensified neoadjuvant chemoradiotherapy (CRT) in rectal cancer has not been proved. We investigated clinical outcomes of intensified 5-fluorouracil plus leucovorin (5-FU/LV) chemotherapy.
Methods
We retrospectively analyzed 45 patients with locally advanced rectal adenocarcinoma who underwent neoadjuvant CRT between 2010 and 2015. Intensified group took additional 1 cycle of 5-FU/LV chemotherapy after radiation completion (resting period) before surgery, compared to conventional group.
Results
Eighteen patients were in conventional group and 27 were in intensified group. Median follow-up duration was 33.7 months (range, 7.8–75.6 months). Complete response rate was 11.4% (5/45). Twelve patients in conventional group and 16 patients in intensified group achieved downstaging (P=0.435). In aspect of toxicity, anemia and thrombocytopenia tended to be more frequent in intensified group without statistical difference. There was also no difference in survival between two groups.
Conclusion
The intensified CRT with additional 1 cycle of 5-FU/LV in rectal cancer revealed no clinical benefit compared to conventional regimen. Considering that the adverse event was minimal and generally acceptable, further research with additional cycles of 5-FU/LV is needed to prove a real benefit of intensified CRT.
INTRODUCTION
One of the standard treatment modality for locally advanced rectal cancer is neoadjuvant chemoradiotherapy (CRT), which might result in tumor downstaging and tumor regression [1]. Furthermore, in some studies, preoperative CRT reduced local recurrence rate and improved overall survival [2,3]. Conventionally, following two cycles of neoadjuvant chemotherapy using intravenous 5-fluorouracil plus leucovorin (5-FU/LV), the surgery is performed 6 to 8 weeks after the completion of long-course radiotherapy. This resting period between the radiotherapy schedule and the surgery is necessary for tumor downstaging and avoidance of the acute reaction caused by radiation [4]. Some institutes have conducted consolidation chemotherapy with different CRT regimens in this resting period with an expectation of downstaging effect as well as providing effective control early to reduce systemic spread [5–7]. However, there is no evidence-based consensus that demonstrates better oncological outcomes. In addition, the clinicians are concerned about the possibility of more chemotherapy-induced toxicities due to the additional cycle of chemotherapy even though most patients are tolerable for 5-FU/LV regimen.
The purpose of this study is to assess the outcomes of intensified 5-FU/LV chemotherapy compared with conventional two cycles of 5-FU/LV chemotherapy in the neoadjuvant CRT setting for locally advanced rectal cancers.
METHODS
Patients
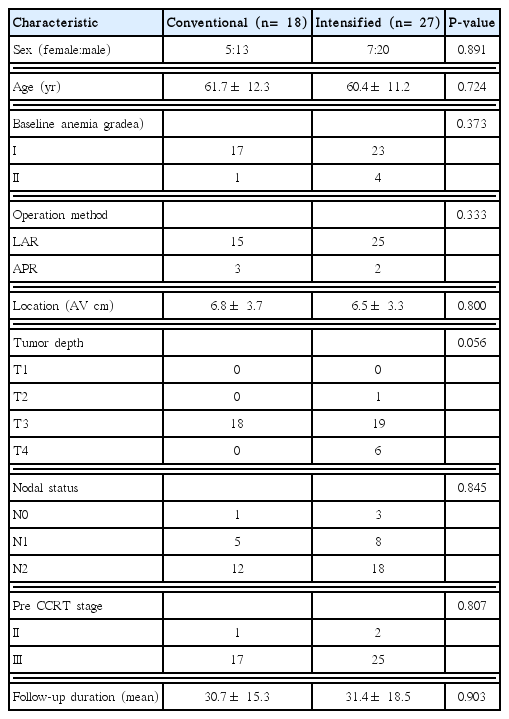
This is a retrospective and single-center analysis with a prospective collected database. This study was approved by the Institutional Review Board of Korea University Medical Center (IRB No. AS16205). Tumor stages were classified on basis of the 7th edition of the American Joint Committee on Cancer tumor-node-metastasis (TNM) grading system. In total, 95 patients with rectal adenocarcinoma underwent neoadjuvant CRT at Korea University Ansan Hospital between January 2010 and December 2015. Inclusion criteria included patients with locally advanced rectal cancers (clinical stage II and III) who underwent curative resection. Patients with two or three cycles of neoadjuvant 5-FU/LV chemotherapy were eligible. Exclusion criteria were stage IV patients (n= 2), other chemotherapy regimens such as oral capecitabine (n= 40), missing data or loss to follow-up (n= 7). Hence, a total of 45 patients were included in this study and their demographic and clinicopathologic data were reviewed.
From 2010, a new strategy has been introduced in our institute which added 1 more cycle of chemotherapy in the resting period between completion of radiotherapy and operation. The former schedule was defined as conventional group and the schedule with additional cycle of chemotherapy was defined as intensified group. Our policy for locally advanced rectal cancers is to perform neoadjuvant CRT, curative surgery and adjuvant chemotherapy. Neoadjuvant CRT consists of 50 Gy of radiation (25 fraction) and concomitant 5-FU/LV infusion (5-FU 425 mg/m2/day and folic acid 20 mg/m2/day) being delivered in the first and last 5 days of radiation (conventional 2 cycles of chemotherapy). Curative surgery was performed 6–8 weeks after radiation completion. Total mesorectal excision was performed for mid and low rectal cancer and a tumor-specific mesorectal excision with a 5 cm distal margin was performed for upper rectal cancer. Within 6 weeks after rectal surgery, patients took adjuvant 4 cycles of 5-FU/LV chemotherapy. Compared to conventional group, intensified group took additional 1 cycle of 5-FU/LV chemotherapy after radiation completion (resting period) before surgery.
Patient evaluation
Pre- and post-CRT evaluation included digital rectal examination; computed tomography scans of the chest, abdomen and pelvis; serum carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA 19-9) levels; magnetic resonance imaging of the pelvis; total colonoscopy or sigmoidoscopy. Post-CRT evaluation was performed 1 or 2 weeks before surgery (6 or 7 weeks after radiation completion).
Pathological complete response (pCR) was defined as the absence of any residual tumor cells detected in the primary rectal lesions or lymph nodes. Adverse events during CRT and additional chemotherapy treatment were recorded using the Common Terminology Criteria for Adverse Events ver. 2.0. Toxicities were assessed at each admission for chemotherapy. The pathological response and downstaging were compared between two groups and the oncological outcome was assessed by the overall survival (OS) rate and the disease-free survival (DFS) rate. OS was defined as the interval in months between the start date of CRT and the date of death from any cause or the date of last visit. DFS was defined as the time between the start date of CRT and the locoregional recurrence after R0 resection, metastasis or progression, or death from any cause.
Statistical analysis
Demographics and clinicopathologic characteristics were compared using 2 test and t-test. Survival rates were analyzed by the Kaplan-Meier method and compared by log rank test. All statistical analysis were performed using SPSS ver. 20 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinical characteristics
Among the 45 patients enrolled in this study, 18 (40%) were in conventional group and 27 (60%) were in intensified group. The characteristics of all patients are summarized in Table 1. The mean age was 61.7 and 60.4 years in conventional and intensified groups, respectively. The mean tumor location was 6.8 and 6.5 cm from anal verge, respectively. In both groups, the proportion of T3 and N2 stage was the largest. Among conventional group, 1 patient was diagnosed as stage II and 17 patients were diagnosed as stage III in pre-CRT period. Among the intensified group, 2 patient as stage II and 25 patients as stage III were diagnosed before treatment. Mean follow-up duration was 30.7 and 31.4 months, respectively.
Response after chemoradiotherapy
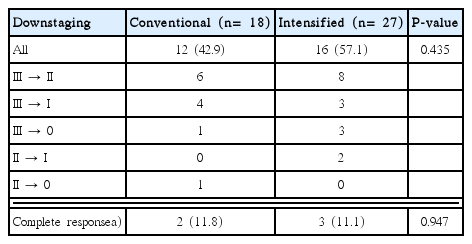
Table 2 shows the pathological response in comparison of initial stage and pathologic stage after operation. Overall, 12 (42.9%) patients in conventional group and 16 (57.1%) patients in intensified group achieved downstaging after CRT (P= 0.435). In conventional group, 11.8% of patients (2/18) achieved a complete response. In intensified group, 11.1% of patients (3/27) had a pCR. Overall, pCR rate of this population was 11.4% (5/45) with no statistically significant difference between two groups (P= 0.947).
Toxicity
Toxicity grading after CRT was shown in Table 3. We assessed two categories, bone marrow suppression and gastrointestinal (GI) trouble, retrospectively. Overall, there was no adverse events of grade IV and over. Anemia grade I tended to be more frequent in intensified group, but there was no statistical difference (P= 0.296). Similarly, more patients in intensified group showed thrombocytopenia grade I without significant difference (P= 0.426). In case of GI toxicity, we reviewed the chart and checked the patients’ complaints of nausea or diarrhea and medications to control the symptoms. There were no significant statistical difference in nausea and diarrhea between two groups (P= 0.134, P= 0.509).
Survival analysis
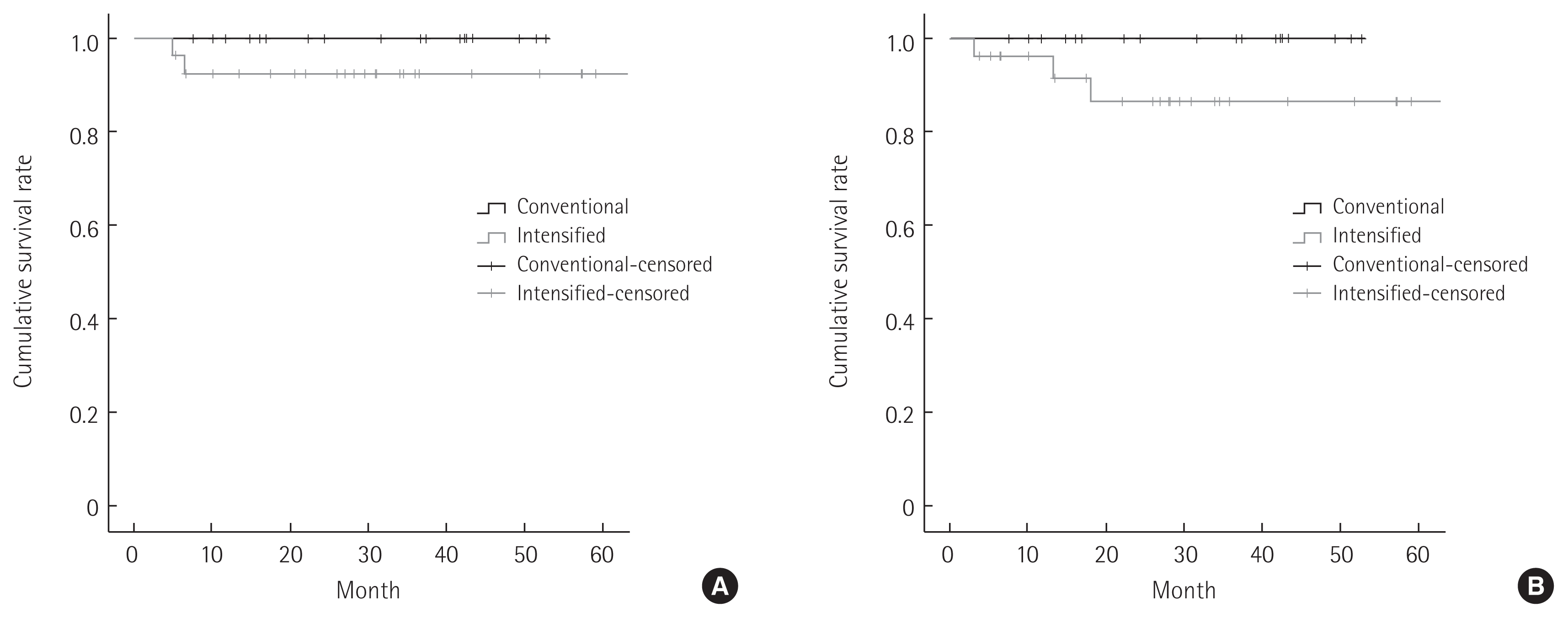
The mean follow-up duration was 31.1± 17.1 months overall. Fig. 1 show OS and DFS curves for conventional group and intensified groups. In Kaplan-Meier curve, the OS rate tended to be better in conventional group than intensified group. However, no statistical difference was observed between two groups (P= 0.239). In addition, the mean DFS of conventional group and intensified group was 34.1 months (range, 7.5–52.7 months) and 29.5 months (range, 4.9–71.9 months), respectively. There was no significant difference in DFS between two groups (P= 0.152).
DISCUSSION
Recently, there has been a remarkable development in various treatment modalities for locally advanced rectal cancer. New alternative regimens for neoadjuvant settings have been tried but to our knowledge, the concept and effectiveness of consolidation chemotherapy in the resting period have not been studied well [6,7].
Clinicians have chosen the intensified regimen in anticipation for better outcome such as tumor downstaging and pCR. However, as shown in this study, the additional cycle of 5-FU/LV during the resting period had no advantage in oncological outcome compared to conventional regimen. Though adding 1 cycle of chemotherapy might be insufficient to obtain clinical benefits, modifications of regimen including additional several cycles can be tried to improve local control and OS. Habr-Gama et al. [6] compared standard CRT and consolidation CRT using a total of 6 cycles of 5-FU/LV chemotherapy, and the authors firstly reported that consolidation therapy could lead to a reduction in tumor metabolic activity assessed by positron emission tomography-computed tomography characteristics (Maximum standardized uptake value). However, the longer resting period can arouse more anxiety for patients and unfavorable cost- effectiveness.
Garcia-Aguilar et al. [5] conducted a multi-institutional trial for locally advanced rectal cancer and showed that adding mFOLF-OX6 between chemoradiation and total mesorectal excision increased the proportion of pCR (38%). However, adding concurrent oxaliplatin to conventional CRT might result in significant increase of toxicities, and incomplete cycles or reduced dose of chemotherapy [5,7].
On that account, the 5-FU based CRT which has low toxicities and high compliance might be chosen for a compromise. During the resting period, both clinician and patient have concern for progression of cancer. Although intensified chemotherapy has no superiority in oncological outcome to conventional regimen, continuity of treatment might relieve the concern. Here, we can design another regimen adding several cycles of 5-FU based chemotherapy not only 1 cycle shown in this study.
The current study has several limitations to be mentioned. First, the retrospective nature, such as selection bias and report variability, might affect the reliability. The standards of pathological assessment and report have changed over time. Second, the small sample size from a single institute was included for the analysis and follow- up duration was rather short to conclude the efficacy. Third, we selected the tumor response (pCR) as an endpoint. However, pCR has not been recommended as an endpoint for long-term survival in the current guidelines [8].
The intensified 5-FU/LV chemotherapy in locally advanced rectal cancer seems to have no superiority in oncological outcome but not much more toxicity rather than conventional regimen. Further studies with larger sample size and a compromise plan such as two or three more additional cycles of 5-FU/LV can be beneficial to validate the effect of consolidation chemotherapy.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.