Taxane-anthracycline combination regimen으로 선행 화학 요법을 시행한 국소 진행성 유방암 환자의 예후예측인자
Prognostic factors in patients with locally advanced breast cancer treated with neoadjuvant taxane-anthracycline combination chemotherapy
Article information
Trans Abstract
Purpose:
The aim of this study is to evaluate the prognostic factors of the group who received neoadjuvant chemotherapy with a taxane-anthracycline combination regimen.
Methods:
We retrospectively analyzed for locally advanced breast cancer patients who received neoadjuvant chemotherapy with adjuvant extension with taxane-anthracycline combination regimen. 101 patients received neoadjuvant chemotherapy with docetaxel and doxorubicin, 46 patients received with epirubicin and paclitaxel. After curative surgery, adjuvant chemotherapy with the same regimen was performed. The adjuvant chemotherapy regimen was adjusted when the response to neoadjuvant chemotherapy was in disease progression.
Results:
The disease free survival is 78% after 5 years and 71% after 10 years for good response group; the disease free survival is 23% in 5 years and 23% in 10 years for the no response group (P<0.001). The overall survival is 93% after 5 years and 90% after 10 years for the good response group; the overall survival is 47% after 5 years and 38% after 10 years for the no response group (P<0.001). 13 patients of 17 patients who to be appeared in complete response were triple negative for breast cancer.
Conclusion:
In order to predict an oncologic outcome from locally advanced breast cancer patients who are treated with neoadjuvant chemotherapy with taxane-anthracycline based regimen, we can use pathologic complete response, an early response to neoadjuvant chemotherapy.
INTRODUCTION
Neoadjuvant chemotherapy (NC) is a standard therapy for locally advanced breast cancer patients [1]. Many studies have reported that there are no differences in the oncologic outcomes between NC and adjuvant chemotherapy [2]. NC provides several advantages, relative to adjuvant chemotherapy. By reducing tumor size, it is possible to perform a breast conserving surgery; also, even if it is possible for the patient to have lumpectomy, by reducing the volume to be removed, the cosmetic effects are improved. Sensitivity of the tumor to chemotherapy are predictable and NC treats micrometastasis earlier. And, there is a report that NC enabled sentinel lymph node biopsy for patients who require axillary dissection during the initial evaluation [3-5].
Generally, tumor size and axillary lymph node metastasis status are the most important prognostic factors for breast cancer patients. When the NC is performed, tumors and axillary lymph nodes are down staged, resulting in the loss of traditional prognostic factors [6]. Other prognostic factors are required for making a difference from adjuvant chemotherapy.
Many studies have reported anthracycline-based regimens increase the benefits compared to cyclophosphamide, methotrexate, and fluorouracil combinations. The taxanes have been used to improve treatment outcomes alone or with anthracycline [7-9].
Schwentner et al. [10] compared the oncologic outcome of the various regimens in adjuvant CTx (cyclophosphamide, methotrexate, and fluorouracil combination, anthracycline-based regimens, taxane-anthracycline-based regimens, dose-dense taxane-anthracycline-based regimens). The N0 and 1–3 positive lymph node metastasis group were not statistically significant different in overall survival (OS) and disease free survival (DFS). In the group with 4–10 positive lymph nodes, the results showed improvement in the oncologic outcomes with taxane-anthracycline-based regimens. In the group of more than 11 lymph nodes, the results showed improvements in the oncologic outcomes with dose-dense taxane-anthracycline based regimens. Many studies reported that the oncologic outcomes have been considered to be the same when the treatments have the same regimen of NC and adjuvant CTx [11]. In locally advanced breast cancer patients, taxane-anthracycline-based regimens are considered to be the better oncologic outcome than the anthracycline-based regimens with neoadjuvant settings.
Several studies evaluated the prognostic factors for breast cancer with different chemotherapy regimens and different patient groups. In 2011, our study reported the prognostic factors in patients treated with an anthracycline-based NC was a clinical response to NC and the absolute number of metastatic axillary lymph nodes [6].
This study evaluated the prognostic factors for locally advanced breast cancer patients who received NC with a taxane-anthracycline combination regimen with an adjuvant extension.
METHODS
Eligibility
This retrospective study includes patients with breast cancer and axillary lymph node metastasis that received NC. Patients received docetaxel+doxorubicin (nTA) from 2001 until 2010 at single center, and patients received paclitaxel+epirubicin (nPE) from 2007 to 2009 in five institutions.
Patients with operable female breast cancer were selected. Other eligibility criteria are Eastern Cooperative Oncology Group performance status scale 0–1, normal left ventricular ejection fraction, adequate hematology (hemoglobin, ≥10 g/dL; absolute neutrophil count, ≥1.5×109/L; and platelets, ≥100×109/L), renal (serum creatinine, ≤1.5 upper normal limits), and liver (aspartate aminotransferase and alanine aminotransferase each of ≤2.5 upper normal limits, and alkaline phosphatase of 5 upper normal limits, total bilirubin of ×1 upper normal limits).
Women who were pregnant, in the lactation period or had distant metastases, accompanied by other malignant tumors were excluded from the study. Staging followed the American Joint Committee on Cancer seventh edition.
Treatment
We confirm breast cancer via core niddle biopsy in the main mass, and the axillary lymph node metastases were confirmed via biopsy, mammography or sonography during the diagnosis period.
nTA was performed with 75 mg/m2 docetaxel and 50 mg/m2 doxorubicin, 3 or 6 times every 3 weeks before operation. nPE was performed with 60 mg/m2 epirubicin and 175 mg/m2 paclitaxel, 4 times every 3 weeks before operation.
Both groups had evaluated responses after 2 cycles of NC. The operations were performed within 3 weeks after the last neoadjuvant CTx, and modified radical mastectomy or breast conserving surgery (lumpectomy, quadrantectomy) with adjuvant radiation followed. If the patients are the criteria on hormone therapy or targeted therapies, those patients received hormone therapy and herceptin therapy after the operation in addition to postoperative chemotherapy.
End point
End point of this study was the OS and DFS. DFS is the period from operation to the first confirmed relapse or the follow-up loss, and the OS is the period from the operation day to the death or follow-up loss.
Tumor response
Tumor response was tested after second cycle of NC. With mammography and sonography, we evaluated the tumor size and compared it with the first diagnosis. The local response to treatment is usually defined following the World Health Organization criteria as follows: complete response (CR), defined as a complete disappearance of all of the known disease and no new lesions; partial response (PR), defined as a 50% reduction in the total tumor load of all measurable lesions; stable disease (SD), defined as a tumor that does not qualify for CR/PR; or, progressive disease (PD), defined as a 25% increase in the size of 1 or more measurable lesions or the appearance of new lesions. When PD occurred as the response result, we changed the regimen after operation. Axillary lymph node response was not evaluated. We defined pathologic complete response (pCR) as when a tumor was not observed and the tumor cells were negative in the axillary lymph nodes.
Statistical aspect
OS and DFS were analyzed via univariate analysis with the Kaplan and Meier method and a log rank test. Multivariate analysis was used to adjust for confounders with Cox’s regression model. Factors identified as affecting DFS and OS by the univariate analysis were entered in Cox’s regression model. The univariate analysis of the clinicopathologic factors with the response between 2 groups was performed with chi-squared test. This study used IBM SPSS ver. 21 (IBM Co., Armonk, NY, USA) for the statistical analysis.
RESULTS
We studied a total of 151 patients, 101 patients of which were treated with docetaxel+doxorubicin (nTA) from 2001 until 2010 at single center. And, 46 patients were treated with paclitaxel+epirubicin (nPE) from 2007 until 2009 at 5 separate institutions.
85 patients received 3 treatments of NC with nTA, 16 patients were treated 6 times with NC including nTA, and 46 patients were treated 4 times with nPE.
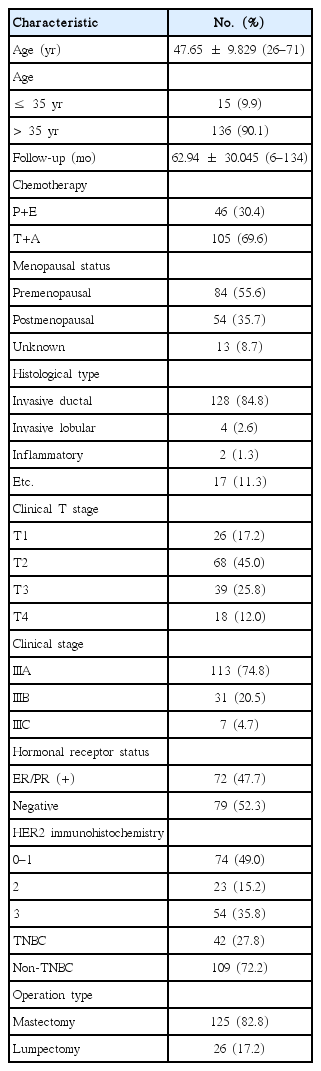
The characteristics of the patients are detailed in Table 1. The mean age was 47.65±9.829 (range, 26–71) and the follow-up mean in months was 62.94±30.045 months (range, 6–134 months).
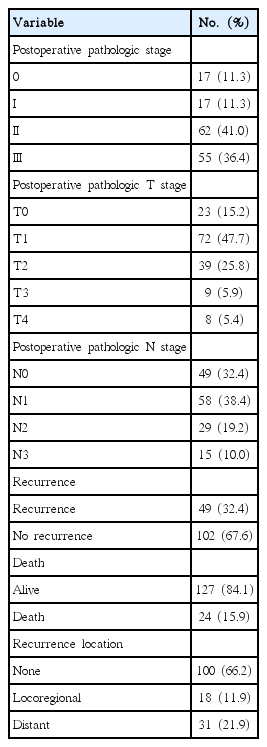
The results after operation evaluated response to NC are detailed at Table 2. Forty-nine patients had recurrence during the follow-up period; 24 patients died because of breast cancer metastasis.
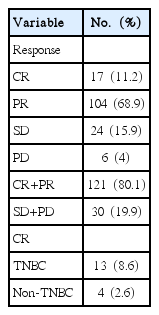
The response to NC is detailed in Table 3. The good response group, CR+PR, had 121 patients, and the no response group, SD+PD, had 30 patients. Thirteen patients out of 17 patients who responded to NC with a complete response were triple negative for breast cancer (estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 all being negative). And, 4 patients were non-TNBC.
The univariate and multivariate analysis of the factors affecting DFS and OS are detailed in Table 4. The univariate analysis showed that the factors affecting DFS were clinical T stage, responsiveness to NC, pathologic stage, pathologic T stage, pathologic N stage, operation type, hormonal receptor status, and HER2 immunohistochemistry. The multivariate analysis showed that the affecting DFS was responsive to NC and the hormonal receptor status. The univariate analysis showed that the factors affecting OS were responsive to NC, pathologic stage, pathologic T stage, operation type, and HER2 immunohistochemistry. The multivariate analysis showed the affecting OS was responsive to NC.
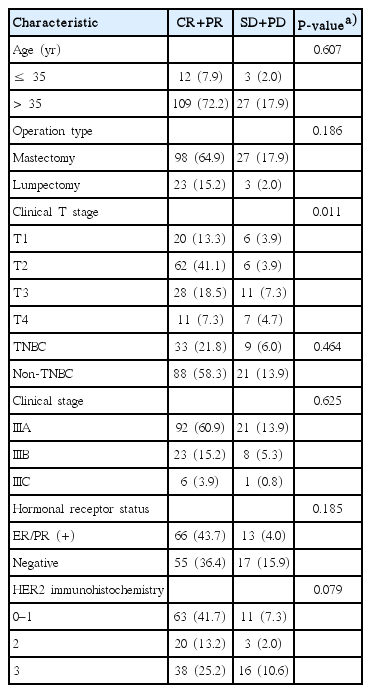
The patients were grouped as CR+PR and SD+PD by their responses to NC. There were no differences between 2 groups, according to their age, operation type, triple negative breast cancer (ER, PR, and HER2 negative), clinical stage, Hormonal receptor status, HER2 immunohistochemistry. The lower clinical T stage, CR+PR had more than SD+PD (Table 5).
The cumulative chance of DFS of CR+PR for 60 months was 0.78; and for 120 months was 0.71. Those in SD+PD for 60 month had a probability of 0.23 with those in the same group at 120 month at 0.23 (P<0.001).
The cumulative chance of OS of CR+PR for 60 months was 0.93, 0.90 for 120 months. Meanwhile, the chance of SD+PD for 60 months survival was 0.47 with a 0.38 chance for 120 months (P<0.001) (Fig. 1).
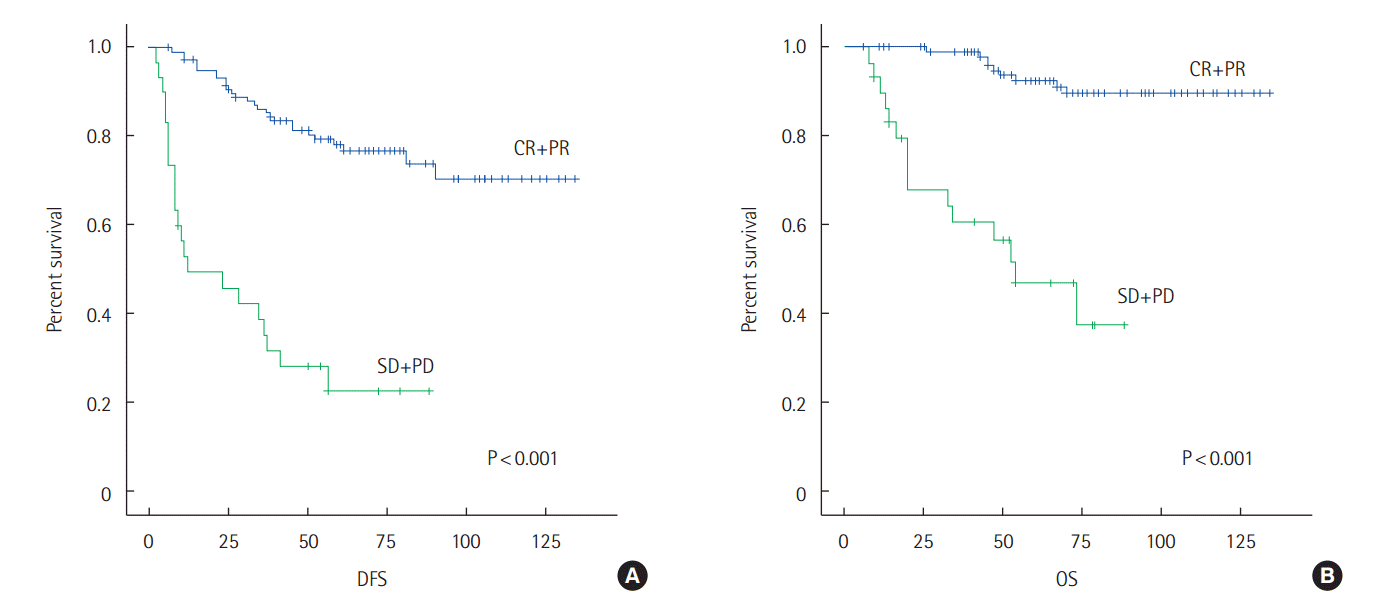
(A) The DFS curve of the CR+PR group and the SD+PD group for breast cancer. The cumulative chances of DFS for CR+PR group are 0.78 for 60 months and 0.71 for 120 months; the cumulative chances of DFS for the SD+PD group are 0.23 for 60 months and 0.23 for 120 months (P<0.001). (B) The OS curve of the CR+PR group and the SD+PD group for breast cancer. The cumulative chances of OS for the CR+PR group are 0.93 for 60 months and 0.90 for 120 months; the cumulative chances of OS for the SD+PD group are 0.47 for 60 months and 0.38 for 120 months (P<0.001). DFS, disease free survival; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; OS, overall survival.
Comparing the oncologic outcomes by hormone receptor (HR) status, ER/PR positive breast cancer patients, the longer DFS period (P=0.008), and there were no differences between any two groups in OS (P=0.071) (Fig. 2).
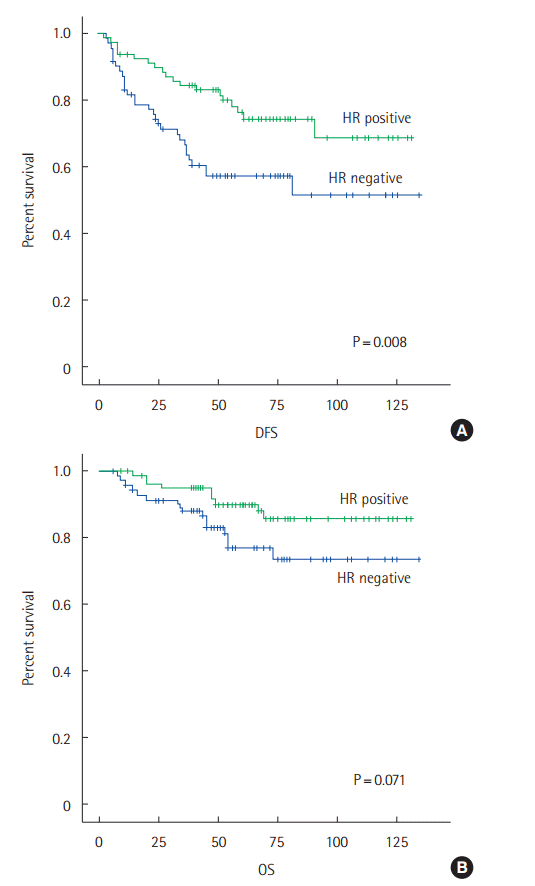
(A) The DFS curve of the HR positive group and the HR negative group for breast cancer. The cumulative chances of DFS for the HR positive group are 0.76 for 60 months and 0.68 for 120 months; the cumulative chances of DFS for the HR negative group are 0.57 for 60 months and 0.51 for 120 months (P=0.008). (B) The OS curve of the HR positive group and the HR negative group for breast cancer. The cumulative chances of OS for the HR positive group are 0.90 in 60 months and 0.86 in 120 months; the cumulative chances of OS for the HR negative group are 0.77 in 60 months and 0.74 in 120 months (P=0.071). DFS, disease free survival; OS, overall survival; HR, hormone receptor.
DISCUSSION
Taxane improves outcomes for breast cancer when used alone or combined with anthracycline and is used as a neoadjuvant and adjuvant setting and in early breast cancer and metastatic cancer [7,9]. Epirubicin, doxorubicin (anthracycline) and paclitaxel and docetaxel (a taxane) are already well-known as the most effective for metastatic breast cancer [12,13].
This study evaluated patients with operable, locally advanced breast cancer who had axillary lymph node metastasis but no distant metastasis when patients were confirmed with the diagnosis of breast cancer [14]. The taxane-anthracycline combination regimen (docetaxel+doxorubicin, paclitaxel+epirubicin) was administered. There was no difference in DFS and OS between the two regimens.
The pathologic response to NC becomes a marker for long-term survival [2,3,15]. Operations after neoadjuvant CTx create down staging, so it makes difficult for post-operative pathologic results to predict prognoses. There have been lots of studies for predicting prognoses [6,16]. In this study, a group with an early response showed better oncologic outcomes than the no response group (DFS P<0.001, OS P<0.001). In order to predict oncologic outcomes after NC, an early good response is a useful prediction factor.
In this study, the factor affecting response to NC is the initial T stage. The lower T stages showed a better response to NC (P< 0.011). Kim et al. [6] reported the same result. And Estevez and Gradishar [16] reported that a smaller tumor is a marker for an improved outcome.
Estevez and Gradishar [16] stated that the main goal of neoadjuvant CTx has to be pCR. pCR is a predicting factor for good outcomes and long survival as an early response for neoadjuvant CTx [17]. In this study, 11.2% (17 among 151 patients) showed pCR. Chollet et al. [18] reported 15% showing pCR, and Kuerer et al. [19] reported a 12% pCR. The rates of pCR may vary according to the definition of pCR. The pCR rate is in the range of 3%–46% [20].
Triple negative breast cancer (TNBC) was recorded in 13 among 17 patients who showed pCR. 13 (30%) had pCR among 42 TNBC, and 4 had pCR among 109 patients. In the report of von Minckwitz et al. [21], 34% of the TNBC group showed pCR. However, in this study, TNBC had no statistical significance in the groups divided as CR+PR and SD+PD. The rest of the 29 patients who did not show pCR may have similar or worse responses as non-TNBC in the response to NC. It is known that TNBC patients group had bad prognoses in the report by von Minckwitz et al. [22]. There are lots of studies on the predicting factors of pCR. Kim et al. [23] reported expression of p53 was associated with pCR with TNBC. Considering high pCR rate in TNBC and the bad prognoses for those in the non-pCR group, it seems that it is necessary to study pCR predicting factors in TNBC and the effective CTx regimens for TNBC.
In this study, the hormone receptor (HR) status was a factor affecting DFS with response to NC. ER/PR positive breast cancer patients have a longer DFS than those with ER/PR negative breast cancer (P<0.001). The OS of ER/PR positive breast cancer patients did not reach statistical significance, but the direction is toward the longer OS (P=0.071). The good prognoses of HR positive breast cancer patients are well known. But, several studies reported NC changed the HR status [24]. In this study, the HR status was evaluated after operation. HR status before NC could not be determined. And, whether the HR status changed after the NC affects outcome is not known. Jin et al. [25] reported a 5.4% change from HR (–) to HR (+), and the loss of HR positivity was shown to be worse for DFS and OS.
In 2011, our study reported prognostic factors in patients treated with anthracycline-based NC. it recorded as prognostic factors a clinical response to NC and an absolute number of metastatic axillary lymph nodes [6]. Many other studies reported that the pN status can be used as another factor predicting prognosis while expecting the loss of traditional prognostic factors in NC response [6,16]. In this study, the pN status showed statistically significant differences in univariate analysis. However, in multivariate analysis, it did not have statistically significant differences in OS and DFS. It seems possible that the pN status will become a prognostic factor of oncologic outcomes, because the N stage may be high before NC, or it is a sign of a bad response to NC. If the tumor response equals the LN response, the tumor response is more important than the pN status for prognostic predictions. Taxane-anthracycline-based regimens had better responses in the high LN status group [10]. Whether the pN status can be used for prognostic predictions needs further research.
In order to predict an oncologic outcome from locally advanced breast cancer patients who are treated with NC with taxane-anthracycline based regimen, we can use pathologic complete response, an early response to NC.
Notes
No potential conflict of interest relevant to this article was reported.