INTRODUCTION
Gallbladder cancer is a rare cancer, accounting for 1.2% of cancer worldwide [1]. In Korea, gallbladder cancer showed an incidence rate of 2.96/100,000 (2009–2013) [2]. Despite its rare incidence, the 5-year survival rate of gallbladder cancer was 28.8% in Korea [3]. This poor outcome is thought to be due to ambiguous symptoms of gallbladder cancer, which delay its diagnosis before it has advanced or metastasized, leading to overall progression [1]. In the case of resectable gallbladder cancer, surgical resection is the only curative treatment option, with perioperative chemotherapy [4]. For unresectable gallbladder cancer, a palliative chemotherapy regimen of gemcitabine and cisplatin is standard of care [4]. However, some patients may suffer from the adverse effects of the anticancer agents, which makes it hard for them to continue the chemotherapy.
Viscum album, also known as European mistletoe, has been used in integrative medicine to enhance the quality of life and to reduce adverse effects in cancer patients [5,6]. It has been studied that V. album extracts have cytotoxicity and immunomodulatory effects, which are presumed to show anticancer activity [7–9]. Effect of V. album has been noticed in several other cancers. Previous studies have shown that V. album may induce prolongation of survival time in cancer patients [10–12]. V. album also improves the quality of life in cancer patients and reduces the adverse effects of conventional chemotherapies [6].
However, no previous study was done to evaluate the effect of V. album on gallbladder cancer. Here, we report a case of recurrent gallbladder cancer after laparoscopic radical cholecystectomy, treated with V. album as a bridge to subsequent palliative chemotherapy. This report was approved by the Institutional Review Board of Severance Hospital (4-2023-0907). The informed consent was waived.
CASE REPORT
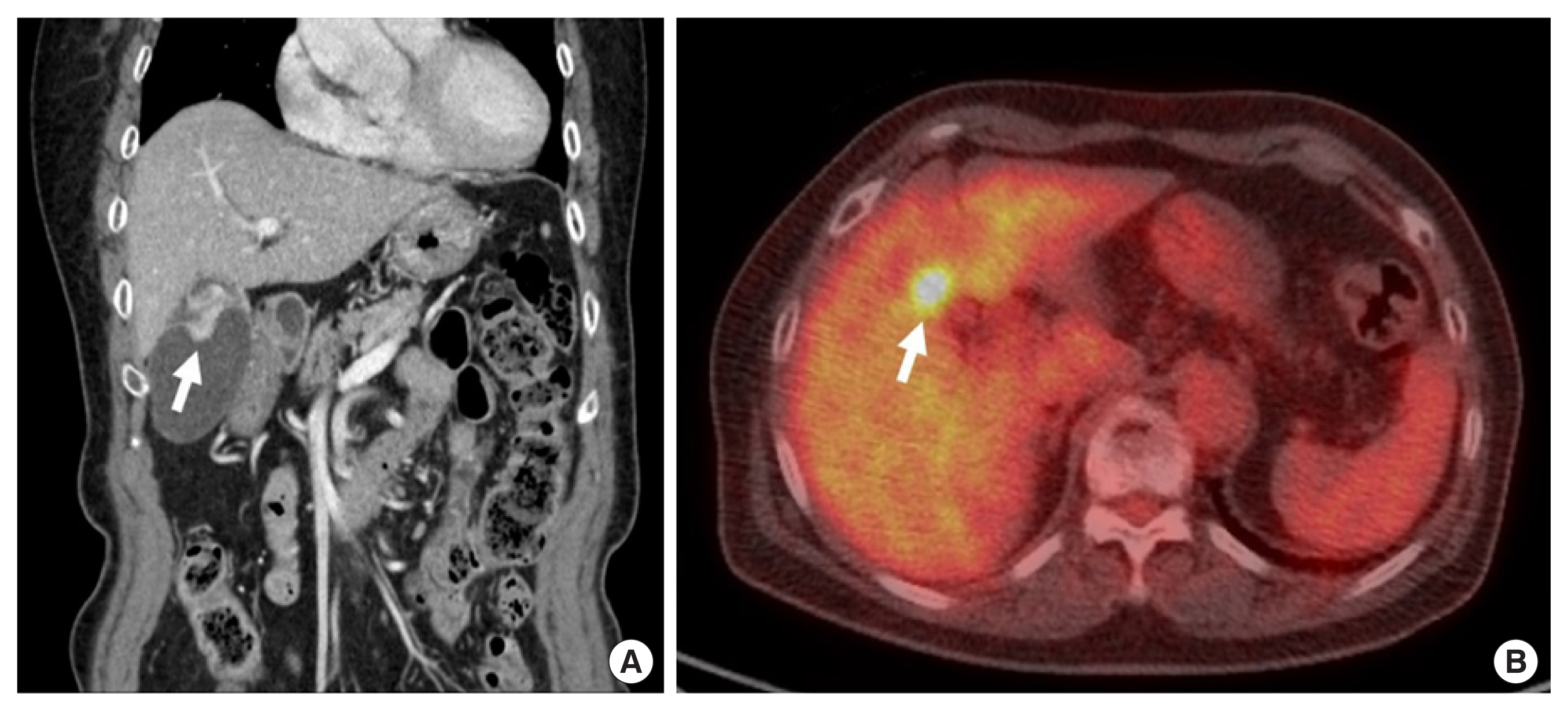
A 78-year-old female patient was admitted for further evaluation and proper management of a mass in the gallbladder body. She had a history of ectopic pregnancy. There was no abnormal finding in review of systems and physical examination. She had no history of smoking and drinking. Preoperative abdomen-pelvic computed tomography (CT) showed a 2.5 cm irregular enhanced wall thickening and luminal protruding mass in gallbladder body with suspicious regional lymph node (LN) metastasis (Fig. 1A). Pancreatobiliary endoscopic ultrasound demonstrated a 33.7 mm-sized hyperechoic mass without posterior shadowing in gallbladder with invasion to perimuscular connective tissue. Mass with increased 18F-fluorodeoxyglucose (F-18 FDG) uptake in gallbladder fundus and LN with FDG uptake in right retroperitoneal space was found on positron emission tomography/CT (PET/CT) (Fig. 1B).
The patient underwent laparoscopic radical cholecystectomy on June 12, 2017. Regional LNs of hepatoduodenal ligament, common hepatic artery, and left gastric artery were dissected. Some of the liver bed was also removed, attached to gallbladder, to reduce gallbladder perforation and exposure. The safety margin was 3.5 cm in cystic duct and 1.2 cm in liver parenchyma. Intraoperative pathology consultation was done with a frozen section of cystic duct margin, which was free from tumor. The operation took 3 hours, the estimated blood loss was 50 mL, and transfusion was not done.
The pathological examination reported that the mass was moderately differentiated adenocarcinoma of gallbladder. Tumor size was about 2.0 cm, and all resection margin was free of carcinoma. Adenocarcinoma invaded perimuscular connective tissue of peritoneal side but not serosa (pT2a). Two regional LNs out of 12 LNs dissected were found to be metastatic (pN1), and there was no sign of distant metastasis (M0). It was pathologically confirmed to be in stage IIIB (pT2aN1M0). Immunohistochemical stain showed CK19(+) on ductal glands of attached bile duct, mutation of p53 absent, HER2(2+). The patient’s postoperative recovery was uneventful. She subsequently received postoperative adjuvant chemotherapy with six cycles of gemcitabine 1,000 mg 1 month after surgery. No evidence of recurrence was seen in follow-up CT during adjuvant chemotherapy. Adjuvant chemotherapy was completed on December 12, 2017.
On May 21, 2018, a follow-up CT showed the interval growth of multiple LNs in left paraaortic and left common iliac area, suggesting recurrent LN metastasis. PET/CT also demonstrated multiple LNs with increased FDG uptake along the portacaval space, paraaortic space, and bilateral common iliac chain, suggestive of metastatic LNs. The carbohydrate antigen 19-9 (CA19-9) level, which was 35.9 U/mL 4 months ago, also showed an elevation to 147 U/mL. The patient was recommended to go through chemotherapy, but she strongly refused due to severe chemotherapy-related toxicity and disappointment. As a result, the recurrent disease was shown to progress continuously.
On May 20, 2019, V. album subcutaneous injection therapy was initiated instead of conventional palliative chemotherapy. Protocol of Abnobaviscum was used to decide the dosage and interval of V. album injection. The 0.02 mg was injected on May 20, 2019. After the initiation of the treatment, a follow-up abdomen-pelvic CT was taken on August 26, 2019, showing a gross interval decrease of multiple metastatic LNs. Abdomen-pelvic CT with contrast done on December 5, 2019, showed further decreased multiple metastatic LNs (Fig. 2). However, on November 6, the patient experienced relapsing fever and dermatitis at the injection site, which are commonly expected side effects of V. album treatment [13]. This occurred after the dosage was increased to 2 mg on October 28. After topical corticosteroid treatment and temporary discontinuation of V. album, dermatitis was effectively managed. The patient resumed V. album therapy with a reduced dose of 0.2 mg on December 2. Notably, no other side effects were reported during the remaining treatment period. CA19-9 level showed a constant and significant decrease during the period of V. album therapy as it decreased from 1,925 U/mL, before the initiation of therapy, to 252 U/mL after the therapy was done for 10 months (Fig. 3). Despite the lack of evidence, we decided to continue V. album therapy until the screening shows evidence of progressive disease.
V. album, as a bridge to subsequent palliative chemotherapy: Abdomen-pelvic CT taken on October 14, 2020 (about 1 and half years after V. album treatment/about 40 months after surgery) showed slightly increased sizes of metastatic lymphadenopathies with CA19-9 level from 317 to 617 U/mL. At this moment, the patient agreed to have second-line palliative chemotherapy, and second chemotherapy with gemcitabine plus cisplatin was initiated on October 26, 2020. Six cycles of gemcitabine plus cisplatin chemotherapy were successfully done, showing stable disease during follow-up (RECIST).
DISCUSSION
Gallbladder cancer is a rare malignancy but is the most common malignancy of the biliary tract. The diagnosis of gallbladder cancer is usually delayed due to its vague symptoms, which is the cause of its poor prognosis. Since gallbladder cancer usually advances at the time it is diagnosed, additional therapy after surgery is important in the treatment of gallbladder cancer.
Recurrent advanced gallbladder cancer is usually managed with chemotherapy. Effective regimens for chemotherapy have been established through investigations, although the current evidence is mainly established for biliary tract cancer in general, which means that there is no specific palliative chemotherapy regimen for gallbladder cancer [4].
Combined chemotherapy with gemcitabine and cisplatin is the first-line therapy, while there is limited evidence for the second-line regimen for chemotherapy for gallbladder cancer [4]. However, because of the adverse effects of chemotherapy, some patients may not be able to continue the treatment. In this case, it was clinically difficult to proceed with the second-line chemotherapy in principle because the patient was elderly and had already undergone the first-line chemotherapy.
In such a case, V. album may be an alternative choice because it is known to be a safe agent with mild adverse effects and improves the quality of life in cancer patients. In this case, V. album may serve as “bridge therapy,” which could modulate cancer until the patient can restart chemotherapy. There is plenty of evidence that V. album has anti-tumor properties, including immunomodulation and cytotoxicity, both in vitro and in vivo [7–10]. However, the oncological effects of V. album have not been proven in randomized controlled trials [14,15]. Pelzer et al. [14] reported that no significant differences in relapse or metastasis were found in patients who have been treated with V. album. Grossarth-Maticek et al. [10] reported that there was no significant effect of long-term V. album therapy on tumor progression. Moreover, the clinical evidence for using V. album for gallbladder cancer is still insufficient.
In this case, after 5 months from the adjuvant chemotherapy with gemcitabine and cisplatin, interval change of abdomen-pelvic CT and CA19-9 showed the asymptomatic recurrence of cancer. Starting chemotherapy again was recommended, but it could not be started as the patient refused it. As an alternative therapy, a subcutaneous injection of V. album was used. During the therapy, follow-up abdomen-pelvic CT showed a decreasing tumor burden, and serum CA19-9 level showed a rapid and significant drop. Though the progression of cancer occurred eventually, the patient stayed progression-free and even regressed for about 17 months, gaining enough time to prepare for chemotherapy. Considering that no other anticancer agents were provided for the patients, this tumor regression phenomenon was thought to be derived from V. album therapy.
To the best of our knowledge, this is the first reported case of usage of V. album in gallbladder cancer. In this case, V. album showed a potential to become a decent “bridge therapy,” not only being an alternative for patients who are not able to use chemotherapy but also modulating the cancer progression until the patients are ready to go through chemotherapy. Further research is needed to investigate the clinical effect of V. album in gallbladder cancer.