ABSTRACTPurposeSeveral studies are concerned about the association between bone mineral density (BMD) and the risk of breast cancer in postmenopausal women, but it is controversial. Therefore, we evaluated whether BMD might be a risk factor for recurrences, or metastases in menopausal luminal A breast cancer patients.
MethodsIn this retrospective study, data of 348 patients with luminal A breast cancer who received treatment at Pusan National University Yangsan Hospital between 2012 and 2016 were analyzed. Patients were divided into two groups: normal BMD and low BMD including osteopenia or osteoporosis in preoperative examination. Patients were also divided into three groups according to BMD changes: no change in BMD; improvement in BMD, and deterioration in BMD. Events were defined as recurrence, occurrence of contralateral breast cancer, and metastasis to any other organ.
ResultsPreoperative examination revealed normal BMD in 129 of 348 patients and low BMD in 219 patients. During a median follow-up period of 78 months, only 14 patients (4.0%) experienced recurrences, distant metastases, or occurrences of contralateral breast cancer. Five-year disease-free survival rate was 98.2% for 219 patients with low BMD and 95.0% for 129 patients with normal BMD (P=0.33). Disease-free survival at 5 years was 97.0% for the no change in the BMD group, 94.6% for the BMD improvement group, and 98.4% for the BMD deterioration group (P=0.79).
INTRODUCTIONHormone receptor-positive (HR+) breast cancer is the most prevalent subtype of breast cancer [1,2]. Estrogen exposure has an impact on risk factors for HR+ breast cancer, including hormone replacement therapy, early menarche, and late menopause [3–5]. Furthermore, as estrogen controls bone turnover, increased bone mineral density (BMD) has been thought to be a sign of persistent estrogen exposure [3,4,6–8]. Therefore, it has been hypothesized that high BMD is associated with a worse prognosis for breast cancer. A previous study has shown that postmenopausal breast cancer patients with low BMD have lower rates of local and distant recurrences than patients with normal BMD [9]. The subtype of breast cancer was not classified in that study, making it difficult to apply its results to all postmenopausal patients with breast cancer. Due to widespread use of aromatase inhibitors, postmenopausal breast cancer patients are especially susceptible to bone density loss. Several studies have demonstrated that aromatase inhibitor-treated breast cancer patients experience an elevated incidence of osteoporotic fractures [10–12]. Therefore, bone density reduction should not be neglected, as previous research has shown that increased bone density is a poor prognostic factor for breast cancer. The objective of the present study was to examine the associations between BMD and breast cancer recurrences among postmenopausal patients with luminal A breast cancer. Associations of breast cancer recurrences with BMD changes during the follow-up period were also evaluated.
METHODSPatientsThis retrospective analysis evaluated 348 postmenopausal patients with luminal A breast cancer who were treated at Pusan National University Yangsan Hospital from 2012 to 2016. Luminal A breast cancer was defined if the following criteria were met: (1) HR positivity; (2) human epidermal growth factor receptor 2 negativity; and (3) Ki-67 percentage less than 15%. Menopause was defined as the absence of menstruation for more than a year combined with elevated follicle-stimulating hormone (>25 IU/mL) levels in the blood. Dual energy X-ray absorptiometry was used to measure BMDs of the lumbar spine, total femur, and femoral neck. According to measured BMD and World Health Organization (WHO) criteria, those with T scores ≥ −1.0, −2.5 to −1.0, and ≤ −2.5 were defined as normal, osteopenia, and osteoporosis patients, respectively. Patients were divided into two groups in the preoperative examination: those with normal BMD and those with low BMD (including osteopenia and osteoporosis). Changes in BMD over the course of the follow-up period were also analyzed according to WHO criteria. The bone density of patients was measured every year after surgery, and it decreased when the change in bone density was lower than before surgery, increased when it improved, and remained unchanged if there was no change. Patients with osteopenia were given calcium medications, while those with osteoporosis were given denosumab. Data such as age at diagnosis, body mass index (kg/m2), histology, tumor size, tumor grade, lymph node status, stage, the type of surgery, chemotherapy, radiation therapy, and endocrine therapy were collected.
Cancers were staged according to the breast cancer anatomic stage guidelines of the 8th American Joint Committee on Cancer. Patient status was monitored every 3 to 6 months for the first 5 years following the initial treatment and then annually thereafter. This study was approved by the Institutional Review Board of Pusan National University, Korea (IRB No. 05-2022-154). Informed consent is not needed in this study.
Statistical analysisMeans of continuous data were compared between those with normal BMD and those with low BMD using the t-test and Mann-Whitney test. Categorical data between two groups were analyzed using the Pearson chi-square test. Events were defined as recurrence in the ipsilateral breast, chest wall or axillary, supraclavicular, infraclavicular, or internal mammary nodes, occurrences of contralateral breast cancer, and metastasis to any other organ. Disease-free survival was calculated from the date of surgery to the date of event. Kaplan-Meier survival function and log-rank test were used to confirm difference in survival rates between the two groups (normal BMD and low BMD). All data were analyzed using SPSS version 23 (SPSS Inc.).
RESULTSAmong 348 patients diagnosed with postmenopausal luminal A type breast cancer, preoperative examination confirmed normal BMD in 129 patients and low BMD in 219 patients. Table 1 summarizes patient and tumor characteristics of the two groups. The normal BMD group had younger patients (55.82±6.86 years) than the low BMD group (61.76±7.95 years) (P<0.001) (Table 1). Body mass index did not differ significantly between the two groups (Table 1). During the treatment and follow-up period, 46 patients in the normal BMD group (35.7%) and 22 patients in the low BMD group (10.0%) showed decrease in BMD (Table 1). All patients received endocrine therapy. In the low BMD group, 47 patients (21.5%) switched from aromatase inhibitor to tamoxifen. In the normal BMD group, only 15 patients (11.6%) made the switch (Table 2). During a median follow-up period of 78 months, only 14 patients (4.0%) had recurrences, distant metastases, or occurrences of contralateral breast cancer. Four patients experienced recurrences: one in the normal BMD group and three in the low BMD group. Six patients were found to have distant metastases: four in the normal BMD group and two in the low BMD group. Contralateral breast cancer was detected in five patients: four in the normal BMD group and one in the low BMD group. Disease-free survival rate at 5 years was 98.2% for 219 patients with low BMD and 95.0% for 129 patients with normal BMD (P=0.33) (Fig. 1). In addition, disease-free survival at 5 years was 97.0% for those whose BMD did not change during treatment, 94.6% for those with BMD improvement, and 98.4% for those with BMD deterioration (P=0.79) (Fig. 2).
DISCUSSIONEstrogen is known to affect bone turnover, and continuous estrogen exposure has been linked to breast cancer [3,4,6]. Thus, it has been hypothesized that high BMD is associated with breast cancer. High BMD has been regarded as a negative prognostic factor for breast cancer. Several studies have found that postmenopausal women with a high BMD have an increased risk of developing breast cancer [3,7,8,13,14]. However, such results could not be applied to all postmenopausal women because previous studies included patients who voluntarily underwent routine health examinations. In addition, because they did not classify subtypes of breast cancer, it was difficult to apply their findings to all patients with breast cancer. In a prospective cohort study, Fraenkel et al. [8] have hypothesized that BMD could serve as a biomarker for breast cancer risk. However, other studies have indicated that there is no correlation between breast cancer and BMD [15–19]. Consequently, although numerous studies have been conducted to clarify this issue, there are no conclusive results. A systemic review has also found that the association between BMD and breast cancer risk is still debatable [20].
This study found no association between BMD and breast cancer recurrence or metastasis (Fig. 1). It also found that bone density change during the follow-up period was unrelated to the prognosis of patients (Fig. 2). Additionally, there were more young women in the normal BMD group than in the low BMD group (Table 1). Therefore, patients with normal BMD are more likely to undergo breast-conserving surgery with radiation therapy than mastectomy. In addition, chemotherapy was administered more frequently in the group with normal BMD. In the low BMD group, there was a higher rate of switching to tamoxifen than in the normal BMD group (Table 2). This might be due to progression of bone density loss and aromatase inhibitor-related side effects such as musculoskeletal pain. After treatment with an aromatase inhibitor, 35.7% of patients in the normal BMD group experienced a decrease in BMD. Moreover, only a few patients showed an increase in BMD during the follow-up period. This decrease in BMD can cause osteoporotic fractures as suggested in previous studies [10–12]. In this study, seven patients with osteoporosis had fractures despite receiving treatment for bone density reduction. Therefore, patients confirmed to have low bone density should be actively treated to prevent fractures.
Due to the small number of patients with events, it was difficult to achieve statistical significance in this study, which exclusively focused on luminal A breast cancer. In addition, when determining how BMD changes affected prognosis, bias was introduced because patients with low BMD were treated with calcium medications to increase BMD. It would be preferred to examine the association between bone density and breast cancer prognosis using multicenter data with a more extended follow-up period.
In conclusion, BMD had no statistically significant associations on recurrences, metastases, or incidences of contralateral breast cancer in postmenopausal patients with luminal A breast cancer. In addition, BMD change during treatment showed no statistically significant associations with breast cancer recurrences, metastases, or contralateral occurrences.
NotesFUNDING
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
REFERENCES1. Kang SY, Kim YS, Kim Z, Kim HY, Kim HJ, Park S, et al. Breast cancer statistics in Korea in 2017: data from a Breast Cancer Registry. J Breast Cancer 2020;23:115-28.
2. Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. Breast J 2009;15:593-602.
3. Howell A, Anderson AS, Clarke RB, Duffy SW, Evans DG, Garcia-Closas M, et al. Risk determination and prevention of breast cancer. Breast Cancer Res 2014;16:446.
5. Samavat H, Kurzer MS. Estrogen metabolism and breast cancer. Cancer Lett 2015;356(2 Pt A):231-43.
6. Zhang Y, Kiel DP, Kreger BE, Cupples LA, Ellison RC, Dorgan JF, et al. Bone mass and the risk of breast cancer among postmenopausal women. N Engl J Med 1997;336:611-7.
7. Hadji P, Gottschalk M, Ziller V, Kalder M, Jackisch C, Wagner U. Bone mass and the risk of breast cancer: the influence of cumulative exposure to oestrogen and reproductive correlates. Results of the Marburg breast cancer and osteoporosis trial (MABOT). Maturitas 2007;56:312-21.
8. Fraenkel M, Novack V, Mizrakli Y, Koretz M, Siris E, Norton L, et al. Bone mineral density in women newly diagnosed with breast cancer: a prospective cohort study. NPJ Breast Cancer 2022;8:21.
9. Zambetti A, Tartter PI. Bone mineral density is a prognostic factor for postmenopausal Caucasian women with breast cancer. Breast J 2013;19:168-72.
10. Lee S, Yoo JI, Lee YK, Park JW, Won S, Yeom J, et al. Risk of osteoporotic fracture in patients with breast cancer: meta-analysis. J Bone Metab 2020;27:27-34.
11. Lee YK, Lee EG, Kim HY, Lee Y, Lee SM, Suh DC, et al. Osteoporotic fractures of the spine, hip, and other locations after adjuvant endocrine therapy with aromatase inhibitors in breast cancer patients: a meta-analysis. J Korean Med Sci 2020;35:e403.
12. Waqas K, Lima Ferreira J, Tsourdi E, Body JJ, Hadji P, Zillikens MC. Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre-and postmenopausal women with early-stage breast cancer. J Bone Oncol 2021;28:100355.
13. Kim BK, Choi YH, Song YM, Park JH, Noh HM, Nguyen TL, et al. Bone mineral density and the risk of breast cancer: a case-control study of Korean women. Ann Epidemiol 2014;24:222-7.
14. Kalder M, Jager C, Seker-Pektas B, Dinas K, Kyvernitakis I, Hadji P. Breast cancer and bone mineral density: the Marburg Breast Cancer and Osteoporosis Trial (MABOT II). Climacteric 2011;14:352-61.
15. Tremollieres F, Ribot C. Bone mineral density and prediction of non-osteoporotic disease. Maturitas 2010;65:348-51.
16. Cauley JA, Song J, Dowsett SA, Mershon JL, Cummings SR. Risk factors for breast cancer in older women: the relative contribution of bone mineral density and other established risk factors. Breast Cancer Res Treat 2007;102:181-8.
17. Kerlikowske K, Shepherd J, Creasman J, Tice JA, Ziv E, Cummings SR. Are breast density and bone mineral density independent risk factors for breast cancer? J Natl Cancer Inst 2005;97:368-74.
18. Stewart A, Kumar V, Torgerson DJ, Fraser WD, Gilbert FJ, Reid DM. Axial BMD, change in BMD and bone turnover do not predict breast cancer incidence in early postmenopausal women. Osteoporos Int 2005;16:1627-32.
Fig. 1Disease-free survival rates in normal bone mineral density (BMD) and low bone mineral density groups. 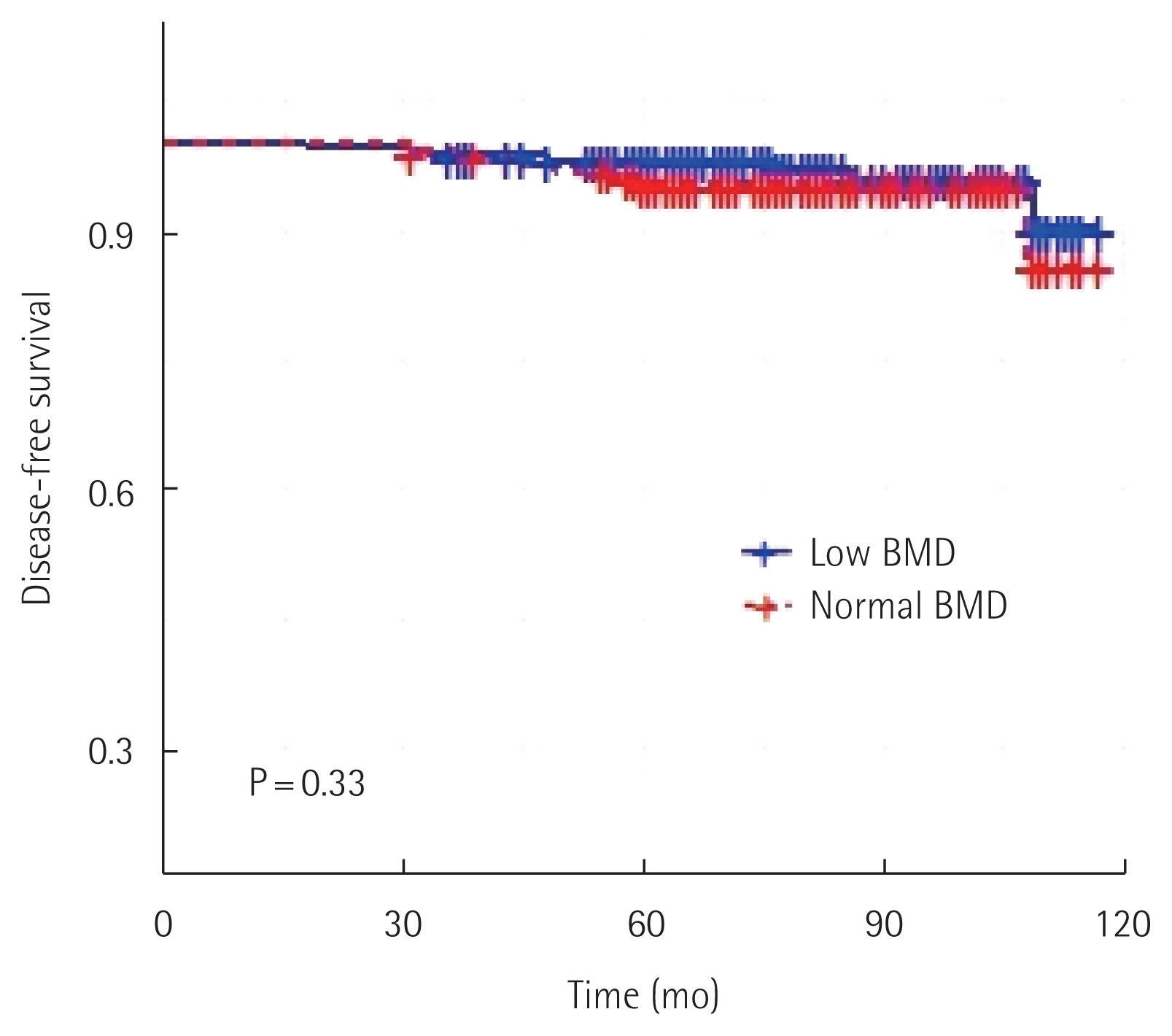
Table 1Patient’s characteristics and pathology according to bone mineral density Table 2Treatments of patients according to bone mineral density |
|
||||||||||||||||||||||||||||||||||||