See commentary "Inflammatory and nutritional markers in patients with resectable pancreatic cancer" in Volume 19 on page 1. ABSTRACTPurposeResectable pancreatic ductal adenocarcinoma (PDAC) has a high risk of recurrence after curative resection; despite this, the preoperative risk factors for predicting early recurrence remain unclear. This study therefore aimed to identify preoperative inflammation and nutrition factors associated with early recurrence of resectable PDAC.
MethodsFrom March 2021 to November 2021, a total of 20 patients who underwent curative resection for PDAC were enrolled in this study. We evaluated the risk factors for early recurrence within 1 year by univariate and multivariate analyses using Cox hazard proportional regression. The cutoff values for predicting recurrence were examined using receiver operating characteristic (ROC) curves.
ResultsIn our univariate and multivariate analyses, C-reactive protein (CRP), CRP-albumin ratio, and CRP-prealbumin ratio, as well as sex and age, were significant independent prognostic factors for early recurrence in PDAC. However, known inflammatory factors (neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios), nutritional factors (albumin, prealbumin, ferritin, vitamin D), and inflammatory-nutritional factors (Glasgow Prognostic Score, modified Glasgow Prognostic Score, albumin-bilirubin) showed no association with early recurrence. In addition, using cutoff values by ROC curve analysis, a high preoperative CRP level of >5 mg/L, as well as high CRP-to-albumin (>5.3) and CRP-to-prealbumin (>1.3) ratios showed no prognostic value.
ConclusionOur results showed that inflammatory and perioperative nutritional factors, especially CRP-to-prealbumin ratio, have significant associations with early recurrence after curative resection in resectable PDAC. Therefore, for such patients, a cautious approach is needed when inflammation and poor nutritional status are present.
INTRODUCTIONPancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy with a poor prognosis; additionally, recurrence after curative resection remains a major challenge in the management of this disease. Despite advances in diagnostic and therapeutic modalities, the 5-year survival rate for PDAC patients after curative resection remains low, at approximately 20% to 25% [1,2]. Although neoadjuvant chemotherapy (NAC) has shown promising results in borderline resectable PDAC, its benefits are controversial [3]. Early recurrence after curative resection is particularly concerning, as upfront surgery is unlikely to be successful. Therefore, identifying reliable predictors of early recurrence in resectable PDAC patients is crucial for optimal treatment planning and improving patient outcomes.
Several prognostic factors such as tumor size, lymph node metastasis, histologic grade, vascular invasion, and perineural invasion have been known to affect outcomes in PDAC [1]. However, these factors have limitations as preoperative predictive factors, as they can only be obtained through postoperative pathologic analyses. Recently, carbohydrate antigen 19-9 (CA19-9) has attracted interest as a prognostic indicator for PDAC, although its usefulness is still debatable [1,4,5]. Although various molecular biomarkers such as gene mutations, exosomes, and miRNA have been identified as potential predictors, there are only a few targets that have scientific bases and practical clinical utility [1,6]. It is also difficult to use them in clinical settings, because discovery of biomarkers is time-consuming, expensive, and resource-intensive. Thus, non-invasive, time-efficient, and cost-effective biomarkers are needed as preoperative predictors of early PDAC recurrence.
Recently, some researchers have focused on the potential role of inflammatory and nutritional factors as preoperative predictors of early recurrence in PDAC [7–10]. Inflammatory and preoperative nutritional factors can be used as relatively simple and accessible preoperative predictors. In this study, we aim to explore the correlation between inflammatory and preoperative nutritional factors and early recurrence in resectable PDAC, and to identify patients who may not be suitable candidates for upfront surgery.
METHODSPatientsTwenty patients who underwent upfront surgery (pancreaticoduodenectomy or radical antegrade modular pancreaticosplenectomy) with resectable PDAC at Pusan National University Hospital between March and December of 2021 were included. Cases with lymph node metastases other than to regional lymph nodes, for which NAC was performed, those who had R2 resections, those with disease recurrence within 3 months post-surgery, and those with other types of malignant diseases (PDAC origin from intraductal papillary mucinous neoplasm, neuroendocrine tumor, mucinous adenocarcinoma, adenosquamous carcinoma and signet ring cell, and other similar malignancies) were excluded. Recurrence within 1 year was defined as early recurrence, and this was found in nine cases. The median duration of follow-up was 15 months. This study was approved by the Pusan National University Hospital Institutional Review Board at the Clinical Trial Center (IRB No. 2303-007-124), and written informed consent was obtained from all participants.
Clinical data collectionThe hemato-chemistry results were based on those performed at the first visit to our center for PDAC evaluation. Pathology results were described by a pathologist specializing in hepatobiliary pancreatic disease. The resection margin status defined a margin of <1 mm as an R1 resection. Preoperative resectability based on chest computed tomography (CT), abdomen CT, magnetic resonance imaging, endoscopic ultrasound, and positron emission tomography-CT was independently determined by three radiologists, three gastroenterologists, and two surgeons who majored in hepatobiliary pancreatic disease. During follow-ups, imaging studies (abdomen and chest CT) and tumor marker tests (CA19-9 and carcinoembryonic antigen [CEA]) were performed every 3–4 months to confirm recurrence. Adjuvant chemotherapy was performed in 17 patients (FOLFIRINOX [15 patients] and gemcitabine and capecitabine [two patients]).
Efficacy of inflammation and nutrition status: CRP-albumin/CRP-prealbuminThe reference ranges of each value were as follows: C-reactive protein (CRP; <0.5 mg/dL), albumin (3.3–5.2 g/dL), prealbumin (20– 40 mg/dL), total vitamin D (25-OH vitamin D2+D3) (>20 ng/mL), ferritin (15–332 ng/mL), CEA (<5 ng/mL), and CA19-9 (<39 U/mL). CRP-to-albumin and CRP-to-prealbumin ratios were used to evaluate the correlation between inflammation-preoperative nutrition and early recurrence. In addition, the area under the receiver operating characteristic curve (AUC) value was obtained using the receiver operating characteristic curve (ROC; cutoff value of CRP-to-albumin ratio: 5.3, AUC: 72.2%; CRP-to-prealbumin ratio: 1.3, AUC: 61.6%).
Statistical analysisClinical and pathological characteristics of the early recurrence and non-recurrence groups were compared and analyzed using the Mann-Whitney U and chi-square tests for continuous and categorical variables, respectively. Univariate and multivariate analyses were conducted using a Cox proportional hazards model. The optimal cutoff values of CRP-to-albumin and CRP-to-prealbumin ratios were determined by analyzing the ROC curves. The AUC was calculated to determine the discriminatory abilities of the indices. Statistical analyses were performed using R software (version 4.2.1). The R packages “moonbook”, “survminer”, “survival”, “plotROC”, “pROC”, and “rocNIT” were used. The statistical significance was set at P<0.05.
RESULTSThe characteristics of the 20 patients with PDAC with early recurrence (n=9) and non-recurrence (n=11) are summarized in Table 1. Age and platelet-to-lymphocyte ratio (PLR) showed statistically significant differences between the two groups (P=0.037 and P=0.056, respectively). Although CRP level (2.7 vs. 0.6, P=0.102), CRP-to-albumin ratio (5.7 vs. 1.6, P=0.119), and CRP-to-prealbumin ratio (1.08 vs. 0.21, P=0.177) tended to be higher in patients with early recurrence than in patients with non-recurrence, the differences were not statistically significant. Other preoperative inflammatory and nutritional factors, such as neutrophil-to-lymphocyte ratio (NLR), PLR; levels of albumin and prealbumin, vitamin D levels, ferritin levels, ALBI grade, Glasgow Prognostic Score, and modified Glasgow Prognostic Score did not exhibit significant differences between the groups. No significant associations were found in terms of other clinicopathological factors such as tumor size, T stage, N stage, metastatic lymph node ratio, lymphovascular invasion, perineural invasion, tumor differentiation, margin status, and levels of CEA and CA19-9 as well.
Table 2 presents the results of a univariate analysis of preoperative factors associated with early recurrence of PDAC using Cox proportional hazards regression. Sex, age, CRP, CRP-to-albumin ratio, and CRP-to-prealbumin ratio were found to be statistically significant prognostic factors for early recurrence. Female patients had a higher risk of recurrence (hazard ratio [HR]=4.094, P=0.038), and older age was also associated with a higher risk of early recurrence (HR=1.086, P=0.049). Higher levels of CRP, CRP-to-albumin ratio, and CRP-to-prealbumin ratio were associated with a higher risk of early recurrence (HR=1.058, P=0.026; HR=1.027, P=0.019; HR=1.156, P=0.010, respectively). Of these, CRP-to-prealbumin ratio was the most significant prognostic factor. In our multivariate analysis, sex, age, CRP level, CRP-to-albumin ratio, and CRP-to-prealbumin ratio were revealed to be related to early recurrence, with HRs of 59.866 (95% confidence interval [CI]= 2.019–1,774.79, P=0.018), 1.353 (95% CI=1.011–1.812, P=0.042), 20.216 (95% CI=1.567–260.754, P=0.021), 0.140 (95% CI=0.026–0.745, P=0.021), and 25.798 (95% CI=1.598–416.38, P=0.022), respectively–suggesting that these factors are independent preoperative predictors for early recurrence in PDAC patients.
Furthermore, the optimal cutoff values of preoperative CRP-to-albumin and CRP-to-prealbumin ratios for predicting early recurrence were set to 5.3 (AUC, 71.2%) and 1.3 (AUC, 61.6%), respectively, by ROC curve analysis (Fig. 1), and patients were divided into two groups according to each cutoff value. Univariate analysis using Cox hazard proportional regression showed that a CRP level of >5 mg/L (HR=7.770, 95% CI=1.857–32.520, P=0.005), a CRP-to-albumin ratio of >5.3% (HR=5.201, 95% CI=1.346–20.100, P=0.017) and a CRP-to-prealbumin ratio of >1.3% (HR=10.380, 95% CI=2.178–49.440, P=0.003) were associated with early recurrence (Table 3). However, in multivariate analysis, these factors based on cutoff values did not show prognostic significance for early recurrence.
DISCUSSIONEven if PDAC is resectable, the overall prognosis remains poor. Although the benefits of borderline resectable PDAC have been established, the debate over whether to perform upfront surgery or NAC in cases of resectable PDAC is ongoing. Surgeons are continuously struggling with the possibilities of missing the timing of surgery due to progression during NAC, or performing unnecessary surgery after early recurrence following upfront surgery. Moreover, PDAC is a very heterogeneous disease, and the uniformity of treatment is still questionable. Thus, by identifying preoperative biomarkers that can detect a high risk of early recurrence after upfront surgery in resectable PDAC, clinicians can better stratify patients, improving their outcomes.
In this study, we found that sex, age, CRP, CRP-to-albumin ratio and CRP-to-prealbumin ratio were significant preoperative predictors of early recurrence in PDAC patients. Although several factors have been identified as potential predictors of early recurrence of PDAC, the relationship between age and sex and PDAC prognosis remains unclear. The reason for this may reflect underlying differences in tumor biology or patient characteristics. One possible explanation for the sex- and age-based differences in recurrence risk is the influence of hormonal and immune factors. Sex hormones such as estrogen and testosterone have been shown to play a role in modulating the immune response [11], and may affect the progression of PDAC. In addition, aging is associated with changes in the immune system, which may contribute to the higher risk of recurrence in older patients [12]. Recently, Xu et al. [13] reported that in the case of older age, poor preoperative nutritional status and slow recovery after upfront surgery prevented or delayed adjuvant treatment, which could affect the prognosis. Due to the small number of enrolled patients in this study, subgroup analysis by age and sex was not possible; thus, further studies are needed to propose a clear reason for this.
Inflammation and nutritional status are closely interrelated in cancer patients. Inflammation plays a critical role in the development and progression of cancer, as well as in the deterioration of nutritional status [14]. Increasing evidence has proven that inflammation markers such as CRP, NLR, and PLR, as well as preoperative nutritional markers such as albumin, prealbumin, ferritin, and vitamin D are known to be related to carcinogenesis and the progression of cancer [15–17]. However, in this study, of these markers, CRP was the only independent predictor of early recurrence in PDAC. CRP, an acute phase marker of inflammation, is mainly synthesized in the liver and induced by proinflammatory cytokines including interleukin-6 and tumor necrosis factor-α, and is commonly elevated in many cancer patients [18]. Albumin and prealbumin are used to assess nutritional status, and are decreased during inflammation due to the transfer of plasma proteins to the reactants of the acute phase, such as CRP [19]. Recent studies have reported that CRP-to-albumin and CRP-to-prealbumin ratios, using the combination of inflammation and preoperative nutritional factors, were significant predictors of various cancer prognoses [20–22]. In this study, not only CRP, but also CRP-to-albumin and CRP-to-prealbumin ratios were found to be independent prognostic factors of early recurrence of PDAC, while other preoperative inflammatory and nutritional factors were not found to have significant associations.
CRP-to-albumin ratio has been reported as a prognostic factor for pancreatic cancer [9,20,23]. Compared to albumin, prealbumin has a shorter half-life of about 2 to 3 days, and is considered to be a more sensitive biomarker for assessing changes in nutritional status over a shorter period of time [24]. Prealbumin levels can decrease rapidly in response to nutritional deficiency or inflammation, which makes it accurate biomarker for monitoring nutritional status. Recent studies have reported that prealbumin is superior to albumin and an independent predictor in cancer patients [24–26]. In our previous study, we found that preoperative low prealbumin levels predicted advanced disease while confirming poor nutritional status in patients with low body mass index and hemoglobin levels who had hepatobiliary pancreatic malignant disease [27]. Based on these findings, the combination of CRP and prealbumin may prove to be a useful biomarker for predicting recurrence and prognosis, but its prognostic value has not yet been elucidated in PDAC patients. This study, to the best of our knowledge, is the first to demonstrate the CRP-to-prealbumin ratio is an independent predictor of early recurrence in PDAC.
In this study, CRP-to-albumin and CRP-to-prealbumin ratios did not show significant prognostic effects when using cutoff values of 5.3 and 1.3, respectively. Other groups have suggested different cutoff values of the CRP-to-albumin ratio for predicting survival and monitoring chemotherapy responses in PDAC patients [20–23]. Estimation of accurate cutoff values needs to be done using large number of patients. It is also important to carefully consider the interpretation of the cutoff value used in each study. Nevertheless, in this study, CRP-to-albumin and CRP-to-prealbumin were still identified as independent prognostic factors for the early recurrence of PDAC. In addition, the relationship between inflammation and preoperative nutritional status may reflect certain aspects of tumor biology. However, several limitations do remain inherent to our study, such as its small sample size and the fact that it was conducted at a single center with a single operator. In addition, the inclusion of PDACs in various locations may have introduced some heterogeneity to our findings.
In conclusion, our study suggests that markers of inflammation and preoperative nutritional status may serve as useful predictors of early recurrence in cases of resectable PDAC, in particular the CRP-to-prealbumin ratio. These findings highlight the importance of evaluating patients’ inflammatory and nutritional statuses prior to deciding on an appropriate treatment approach. In cases with high risks of early recurrence, NAC could be considered instead of upfront surgery. Further studies are needed to confirm these findings, and to identify optimal treatment strategies for patients with resectable PDAC.
NotesFUNDING
This work was supported by clinical research grant from Pusan National University Hospital in 2023.
REFERENCES1. Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378:607-20.
2. Seppanen H, Juuti A, Mustonen H, Haapamaki C, Nordling S, Carpelan-Holmstrom M, et al. The results of pancreatic resections and long-term survival for pancreatic ductal adenocarcinoma: a single-institution experience. Scand J Surg 2017;106:54-61.
3. Seufferlein T, Ettrich TJ. Treatment of pancreatic cancer-neoadjuvant treatment in resectable pancreatic cancer (PDAC). Transl Gastroenterol Hepatol 2019;4:21.
4. Azizian A, Ruhlmann F, Krause T, Bernhardt M, Jo P, Konig A, et al. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep 2020;10:1332.
5. Binicier OB, Pakoz ZB. CA 19-9 levels in patients with acute pancreatitis due to gallstone and metabolic/toxic reasons. Rev Assoc Med Bras (1992) 2019;65:965-70.
6. Giannis D, Moris D, Barbas AS. Diagnostic, predictive and prognostic molecular biomarkers in pancreatic cancer: an overview for clinicians. Cancers (Basel) 2021;13:1071.
7. Fu YJ, Li KZ, Bai JH, Liang ZQ. C-reactive protein/albumin ratio is a prognostic indicator in Asians with pancreatic cancers: a meta-analysis. Medicine (Baltimore) 2019;98:e18219.
8. Zang Y, Fan Y, Gao Z. Pretreatment C-reactive protein/albumin ratio for predicting overall survival in pancreatic cancer: a meta-analysis. Medicine (Baltimore) 2020;99:e20595.
9. Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017;24:561-8.
10. Lu J, Xu BB, Zheng ZF, Xie JW, Wang JB, Lin JX, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer 2019;22:536-45.
11. Orzołek I, Sobieraj J, Domagała-Kulawik J. Estrogens, cancer and immunity. Cancers (Basel) 2022;14:2265.
12. Foster AD, Sivarapatna A, Gress RE. The aging immune system and its relationship with cancer. Aging health 2011;7:707-18.
13. Xu Y, Zhang Y, Han S, Jin D, Xu X, Kuang T, et al. Prognostic effect of age in resected pancreatic cancer patients: a propensity score matching analysis. Front Oncol 2022;12:789351.
14. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol 2014;15:e493-503.
15. An X, Ding PR, Li YH, Wang FH, Shi YX, Wang ZQ, et al. Elevated neutrophil to lymphocyte ratio predicts survival in advanced pancreatic cancer. Biomarkers 2010;15:516-22.
16. Aliustaoglu M, Bilici A, Seker M, Dane F, Gocun M, Konya V, et al. The association of pre-treatment peripheral blood markers with survival in patients with pancreatic cancer. Hepatogastroenterology 2010;57:640-5.
17. Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol 2015;41:1508-14.
18. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448-54.
19. Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med 2002;40:1281-91.
20. Fan Z, Fan K, Gong Y, Huang Q, Yang C, Cheng H, et al. The CRP/albumin ratio predicts survival and monitors chemotherapeutic effectiveness in patients with advanced pancreatic cancer. Cancer Manag Res 2019;11:8781-8.
21. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-reactive protein/albumin ratio, a novel inflammation-based prognostic score, predicts outcomes in patients with hepatocellular carcinoma. Ann Surg Oncol 2015;22:803-10.
22. Liu X, Sun X, Liu J, Kong P, Chen S, Zhan Y, et al. Preoperative C-reactive protein/albumin ratio predicts prognosis of patients after curative resection for gastric cancer. Transl Oncol 2015;8:339-45.
23. Murakawa M, Yamamoto N, Kamioka Y, Kamiya M, Kobayashi S, Ueno M, et al. Clinical implication of pre-operative C-reactive protein-albumin ratio as a prognostic factor of patients with pancreatic ductal adenocarcinoma: a single-institutional retrospective study. In Vivo 2020;34:347-53.
24. Unal D, Orhan O, Eroglu C, Kaplan B. Prealbumin is a more sensitive marker than albumin to assess the nutritional status in patients undergoing radiotherapy for head and neck cancer. Contemp Oncol (Pozn) 2013;17:276-80.
25. Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg 2012;36:2853-7.
Fig. 1The receiver operating characteristic curves and area under curve (AUC) of C-reactive protein (CRP)-to-albumin ratio (A) and CRP-to-prealbumin ratio (B) for early recurrence. The optimal cutoff values were 5.3 (specificity 90.9%, sensitivity 55.6%) and 1.3 (specificity 100%, sensitivity 44.4%), respectively. 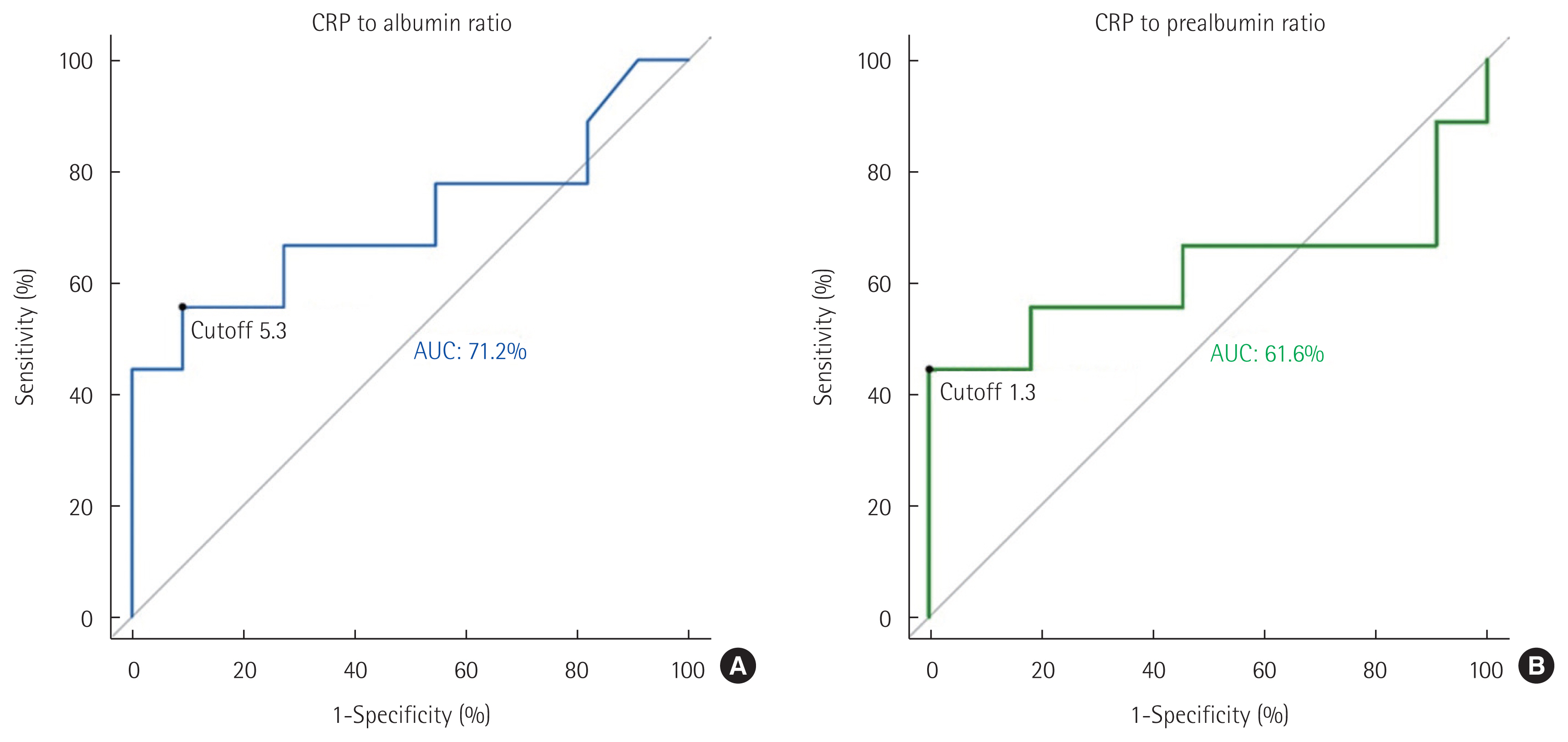
Table 1Characteristics of 20 PDAC patients with early recurrence and non-recurrence
PDAC, pancreatic ductal adenocarcinoma; IQR, interqurtile range; LN, lymph node metastasis; LVI, lymphovascular invasion; PNI, perineural invasion; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI, albumin-bilirubin; GPS, Glasgow Prognostic Score; mGPS, modified GPS; NA, not applicable. Table 2Univariate and multivariate analyses of preoperative risk factors for early recurrence
HR, hazard ratio; CI, confidence interval; LN, lymph node metastasis; LVI, lymphovascular invasion; PNI, perineural invasion; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CRP, C-reactive protein; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALBI, albumin-bilirubin; GPS, Glasgow Prognostic Score; mGPS, modified GPS; NA, not applicable. Table 3Univariate and multivariate analyses of risk factors based on cutoff value for early recurrence
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||