INTRODUCTION
Colorectal cancer is the second most common gastrointestinal malignancy in Korea, and its incidence continues to steadily increase [1]. The prognosis of colorectal cancer is predicted by clinicopathologic stage and one major prognostic factor is lymph node (LN) status, which plays a vital role in determining who benefits from adjuvant chemotherapy. Although localized colorectal cancers with node negative disease are considered potentially curable by surgical resection alone, a 25% rate of recurrence suggests the possibility of occult disease due to suboptimal surgical dissection or pathologic nodal staging [2].
Micrometastasis is defined as small clusters of tumor cells measuring more than 0.2 mm but less than 2.0 mm [3], but its prognostic significance in colorectal cancer is still debatable [4–13]. Because micrometastasis is not easily detected by conventional hematoxylin and eosin (H&E) staining, immunohistochemical staining (IHC) or reverse transcriptase polymerase chain reaction (RT-PCR) can be used. However, from an economic point of view, these special techniques for all harvested LNs are not cost-effective. Thus, focused pathologic examination for a few sentinel LNs is required for clinical application [14].
In the previous study, we examined micrometastasis of tumor-negative LNs in patients with stage I and II colon cancer, and revealed immune-stained neoplastic cells in 5.0% of our patients [15]. These occult tumor cells were not found to have a significant effect on disease-free or overall survival, but all were equally detected by both IHC and H&E re-stained sections. We estimate that one more section on conventional H&E staining would upstage pN0 to pN1 in 5% of node-negative colon cancer patients. Thus, we aimed to examine micrometastasis within tumor-negative LNs in patients with stage I and II rectal cancer by H&E and IHC staining.
METHODS
Patients
We conducted a retrospective cohort study using a prospectively collected institutional colorectal database. The study was approved by the Institutional Review Board. Between December 1995 and November 2010, 180 patients that met the following inclusion criteria underwent resection for rectal cancer; (1) rectal carcinoma proven by biopsy (colon cancer and rectosigmoid colon cancer were excluded) and treatment by curative resection; (2) R0 resection (on clinical and pathologic examinations); (3) stage I or II disease (any T with no lymph node or distant metastasis); (4) no other synchronous malignant tumor; (5) carcinoma not arising from familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer, ulcerative colitis, or Crohn's disease. One hundred twenty-eight (71.1%) men and fifty-two (28.9%) women were included in this study. The median age of patients was 61 years (range, 30–87 years) (Table 1).
Table 1.
Clinicopathologic characteristics of stage I and II rectal cancer
Forty-eight patients received 45–50.4 Gy preoperative radiotherapy in 1.8 Gy fractions. Chemotherapy was administered intravenously and consisted of 5-fluorouracil (5-FU; 425 mg/m2/day) and leucovorin (20 mg/m2/day) during the first and fifth weeks of radiotherapy. Surgical resection, low anterior resection (LAR), intersphincteric resection (ISR) or abdominoperineal excision (APE) was performed 6 to 8 weeks after completion of radiotherapy.
H&E and immunohistochemical evaluation
Original histologic slides of tumor tissues were reviewed by an experienced pathologist. Two thousand two hundred fifty-seven LNs from 180 patients were examined (mean, 12.5 LNs/patient). For each LN, two new slices of 5 μm thickness were obtained from original paraffin blocks. Sections were deparaffinized in xylene and rehydrated. One section was stained with H&E and the other was subjected to immunohistochemistry (IHC). IHC staining procedures were conducted using an automated immunostainer (Vision Biosystems, Vic, Australia). Sections were incubated at a dilution of 1:400 with monoclonal antibody to anti-cytokeratin 20 (CK20; clone K 20.8, Dako, Carpentaria, CA, USA). Immunostaining was developed using 3,3’-diaminobenzidine as chromogen. Appropriate positive controls were added to each automated IHC run to confirm the sensitivity and specificity of the antibody (sections of CK20-positive CRC tissue served as positive controls). The immunostained slides were evaluated by a pathologist who had no knowledge of pathologic data or patient's outcome.
RESULTS
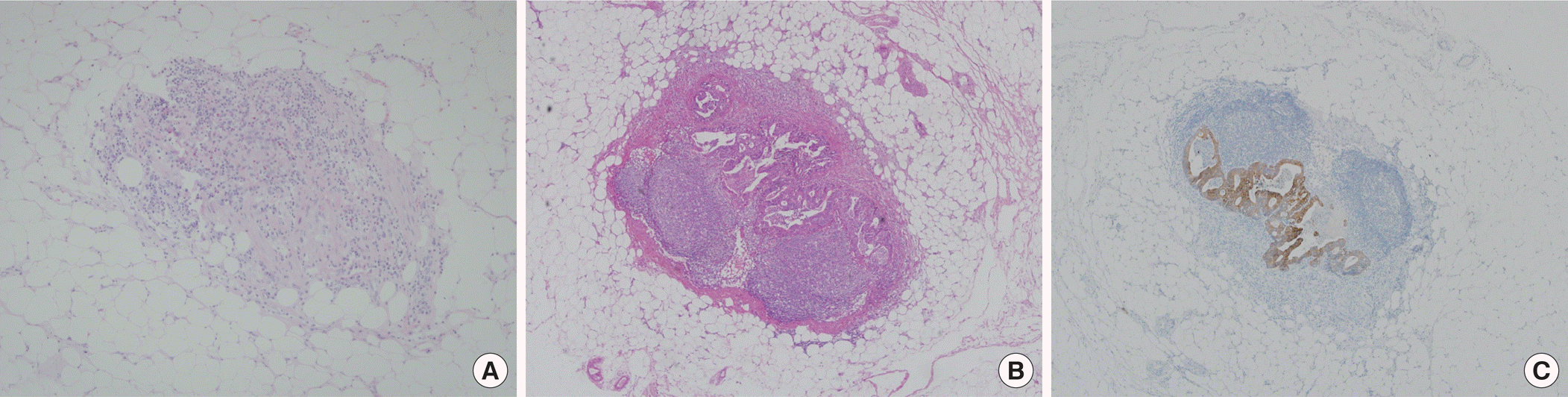
The clinicopathologic characteristics of patients are listed in Table 1. The mean number of harvested LN per patient was 12.5 (range, 1–54). The original H&E slides for 2,257 LNs from 180 patients were reviewed by an experienced pathologist and there was no false negative finding. For IHC staining, CK20-positive neoplastic cells were found in 4 of the 2,257 LNs (0.2%) from 3 of the 180 patients (1.7%), and all corresponding H&E re-stained sections confirmed that these neoplastic cells were present (Table 2). Three of four neoplastic cells were micrometastasis, and one was macrometastasis, resulting in upstage to pN1. Thus, all occult tumor cells were equally detected by both IHC and H&E re-stained sections (Fig. 1). Isolated tumor cells (<0.2 mm) were not found. All occult neoplastic cells were found in stage II disease and were detected in 4 of the 1,197 LNs (0.3%) from 3 of the 85 patients (3.5%).
DISCUSSION
The five-year recurrence rate of patients with node-negative rectal cancer is approximately 20%–30%. Among such a heterogenous group of patients, it is very important to identify high-risk subgroups that will benefit from adjuvant therapy. It is well known that a poorly differentiated tumor, lymphatic and venous invasion, extension to adjacent organs, and inadequate regional LN retrieval all have adverse effects [16]. As a prognostic factor, the role of LN micrometastases in colorectal cancer is still controversial. Some studies have concluded that micrometastasis adversely affects survival of patients compared to node-negative disease [7,8,12], whereas others have failed to demonstrate such a correlation [4–6,9–11,13]. In the present study, recurrence-related or survival data were not evaluated because too few micrometastases were detected and our previous study in patients with colon cancer failed to show the prognostic impact of micrometastasis. We estimated stronger prognostic factors might overwhelm micrometastasis in rectal cancer, which has a higher recurrence rate and different pattern of lymphatic drainage due to pelvic anatomy.
Literature was reviewed for published studies with micrometastasis in rectal cancer on Pubmed and were summarized in Table 3. Studies lacking rectal cancer were excluded. The detection rate of micrometastasis ranges from 7% to 62% [8,17–22]. However, each study employed different patient populations and detection methods. The number of section used for the analysis was different. The rate of detected LN micrometastases increases as the slice number is increased [11]. Studies using only one section for IHC staining showed the detection rates ranging from 17% to 47%, and each study that used four and five sections showed 46% and 62%, respectively. In the present study, IHC stained-neoplastic cells were found in only 3 of the 180 patients (1.7%), suggesting IHC of all harvested LN sections was unhelpful in terms of cost-effectiveness. However, IHC and H&E stained sections were equally sensitive, suggesting that the examination of H&E stained section by a competent pathologist may replace IHC staining and one more section for conventional H&E staining may increase the accuracy of nodal staging or detect micrometastasis in node-negative rectal cancer.
Table 3.
Studies on micrometastasis in rectal cancer
| Study | Neo RT | Stage | Sample size | Harvested LN per patient (mean) | No. of section |
Micrometastasis rate |
Method | |
|---|---|---|---|---|---|---|---|---|
| Patients (%) | LN (%) | |||||||
| Perez et al. [20] | Yes | II | 56 | 9.6 | 3 | 7 | 1.4 | IHC |
| Rosenberg et al. [21] | No | I, II | 28 | 25 | RT-PCR 30 (IHC 4, H&E 2) | 46 | - | RT-PCR |
| Noura et al. [18] | No | II | 29 | 5.5 | (IHC 5, H&E 1) | 31 (IHC 62) | - | RT-PCR |
| Oberg et al. [19] | Yes/no | A, B | 51 | 3 | 1 | 47 | - | IHC |
| Isaka et al. [8] | No | B | 42 | 15.3 | 1 | 21 | 2.9 | IHC |
| Shimoyama et al. [22] | No | I, II | 66 | 13.5 (Lateral LNs) | (IHC 1, H&E 1) | 19 | 2.7 | IHC |
| Fleming et al. [17] | No | A, B | 56 | 14 | 1 | 17.8 | 1.9 | IHC |
Despite studies using RT-PCR showing a higher detection rate of micrometastasis [19,21], they did not use all individual LNs in each resected specimen, but included only peritumoral or pericolic LNs. Such a focused analysis as the examination for peritumoral or sentinel LNs are more likely to detect micrometastasis, but does not represent the true incidence of micrometastasis [14]. Rosenberg et al. [21] using RT-PCR for peritumoral LNs detected micrometastasis in 10 of 22 (45%) patients with stage I colorectal cancer, and Fleming et al. [17] reported that micrometastasis was found in 2 of 14 (14%) patients with Dukes A rectal cancer. In the present study, however, small numbers of occult tumor cells were detected and all belonged to stage II disease. Similarly, in our previous study for patients with colon cancer, all micrometastases were found in stage II disease alone [15].
In summary, we observed a 3.5% rate of occult neoplastic cells from 85 patients with stage II rectal cancer by IHC staining. Interestingly, the results of IHC staining corresponded with those of H&E re-stained sections, suggesting that the examination of H&E stained section by a competent pathologist may replace IHC staining. Large prospective studies are required to address the incidence and prognostic significance of micrometastasis in colorectal cancer.