INTRODUCTION
Despite a significant progress in the management of chemotherapy-induced nausea and vomiting (CINV), it is still considered to be among the most severe and feared adverse effect of chemotherapy.
Aprepitant (EMEND; Merck Co., Whitehouse Station, NJ, USA) is a selective antagonist of the NK1 receptor [1]. Aprepitant in combination with 5HT3-receptor antagonist and dexamethasone has been shown to provide a superior protection in both the acute and delayed phases of CINV during a single cycle of treatment, compared with standard therapy alone [2]. The addition of aprepitant was generally well tolerated and provided consistently superior protection against CINV in patients with moderately emetogenic anthracyclin and cyclophosphamide and also resulted in significantly improved prevention of CINV for patients receiving moderately emetogenic chemotherapy (MEC) [2,3]. In 2006, the Food and Drug Administration (FDA) approved the new indication for oral aprepitant in the prevention of nausea and vomiting associated with initial and repeat courses of MEC [4]. According to the current FDA approved labeling, oral aprepitant is to be given for three days as part of a 3-drug regimen that includes a corticosteroid and a 5HT3-receptor antagonist [5]. The Centers for Medicare & Medicaid Services (Baltimore, MD, USA) proposed to expand coverage in section of the Medicare National Coverage Determinations Manual to include: the oral three-drug regimen of aprepitant, 5HT3-receptor antagonist and dexamethasone is reasonable and necessary for beneficiaries receiving MEC immediately before and within 48 hours after the administration of the anticancer treatment [4]. However, the use of aprepitant is not covered by the medical insurance in Korea at present because there have been fewer studies about the effectiveness of aprepitant in the first cycle of MEC.
The aim of this study was to determine whether aprepitant shows effectiveness for preventing CINV in the first cycle of MEC in colon cancer patients.
METHODS
Patients
We performed a review of a retrospectively maintained institutional database between January 2010 and December 2011. Of the 237 patients who received their first chemotherapy (FOLFOX4, mFOLFOX6 or FOLFIRI regimen) for histological-confirmed colon cancers, 131 patients were included in the present study. One hundred and six patients were excluded by the following criteria: a history of radiation therapy to the abdomen or pelvis within one month before the start of chemotherapy; vomiting within 24 hours before the start of chemotherapy; severe heart, kidney or liver disease (aspartate aminotransferase >2.5 upper limit of normal, alanine aminotransferase >2.5 upper limit of normal, bilirubin >1.5 upper limit of normal, or creatinine >1.5 upper limit of normal); active infection; other factors associated with emesis including brain tumor, brain metastasis, gastrointestinal obstruction, active gastrointestinal bleeding; hypersensitivity to aprepitant, 5HT3-receptor antagonist, dexamethasone; pregnant women and nursing mothers; medication (terfenadine, cisapride, astemizole, pimozide, clarithromycin, ketoconazole, itraconazole, phenytoin, carbamazepine, rifampicin, rifabutin) [5].
Among the total of 131 people, 81 patients received aprepitant in combination with 5HT3-receptor antagonist and dexamethasone (aprepitant group) and 50 patients received 5HT3-receptor antagonist and dexamethasone without aprepitant (control group) in the first cycle of MEC.
Chemotherapy regimen
One of the following chemotherapy agents was administered intravenously (ⅳ): FOLFOX4, mFOLFOX6 or FOLFIRI. (FOLFOX4: Leucovorin200 ㎎/㎡ ⅳ over 2 hours before 5-FU, day 1 and 2 and 5-FU 400 ㎎/㎡ ⅳ bolus and then 600 ㎎/㎡ ⅳ over 22 hours, day 1 and day 2 and Oxaliplatin 85 ㎎/㎡ ⅳ day 1. It consists of 12 cycles, every 2 weeks. mFOLFOX6: Leucovorin 400 ㎎/㎡ ⅳ over 2 hours before 5-FU day 1 and 5-FU 400 ㎎/㎡ ⅳ bolus day 1 followed by 2,400 ㎎/㎡ ⅳ over 46 hours and Oxaliplatin 85 ㎎/㎡ ⅳ day 1. It consists of 12 cycles, every 2 weeks. FOLFIRI: Leucovorin 400 ㎎/㎡ ⅳ over 2 hours before 5-FU day 1 and 5-FU 400 ㎎/㎡ ⅳ bolus day 1, and then 2,400 ㎎/㎡ ⅳ over 46 hours and Irinotecan 180 ㎎/㎡ ⅳ over 90 minutes day 1. It is administered every 2 weeks).
Assessments
On day 1 and throughout the study, we analyzed the results of the complete response, defined as no vomiting and no use of rescue therapy, and the incidence of adverse events during the 120 hours postchemotherapybetween the two groups. We assessed complete response in the acute phase (0-24 hours after initiation of the first cycle of chemotherapy), delayed phase (24-120 hours after initiation of the first cycle of chemotherapy) and overall phase (0-120 hours after initiation of the first cycle of chemotherapy). The rescue therapy, defined as any medication taken to treat established nausea or emesis, was also recorded.
Tolerability was monitored by physical examinations including vital signs and weight measurement, laboratory studies, and electrocardiographys, as well as by adverse events. The investigator determined whether any adverse events were possibly or definitely related to the study drug.
Statistical analysis
The collected variables were analyzed for significant differences between the two groups. The Fisher exact test, chi-square test, and independent sample t-test were used where appropriate. The Kaplan- Meier curve and relative log-rank tests were used to demonstrate percentages of patients without emesis during acute, delayed and overall cycle after initiation of the first cycle of chemotherapy. A P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULT
Patients
Patients’ baseline demographics are listed in Table 1. Among 131 patients, 81 patients are in the aprepitant group and 50 patients are in the control group. Both treatment groups were similar with respect to basic characteristics. The majority of patients were male (51/131, 61%) and an average age of 60 years.
Efficacy
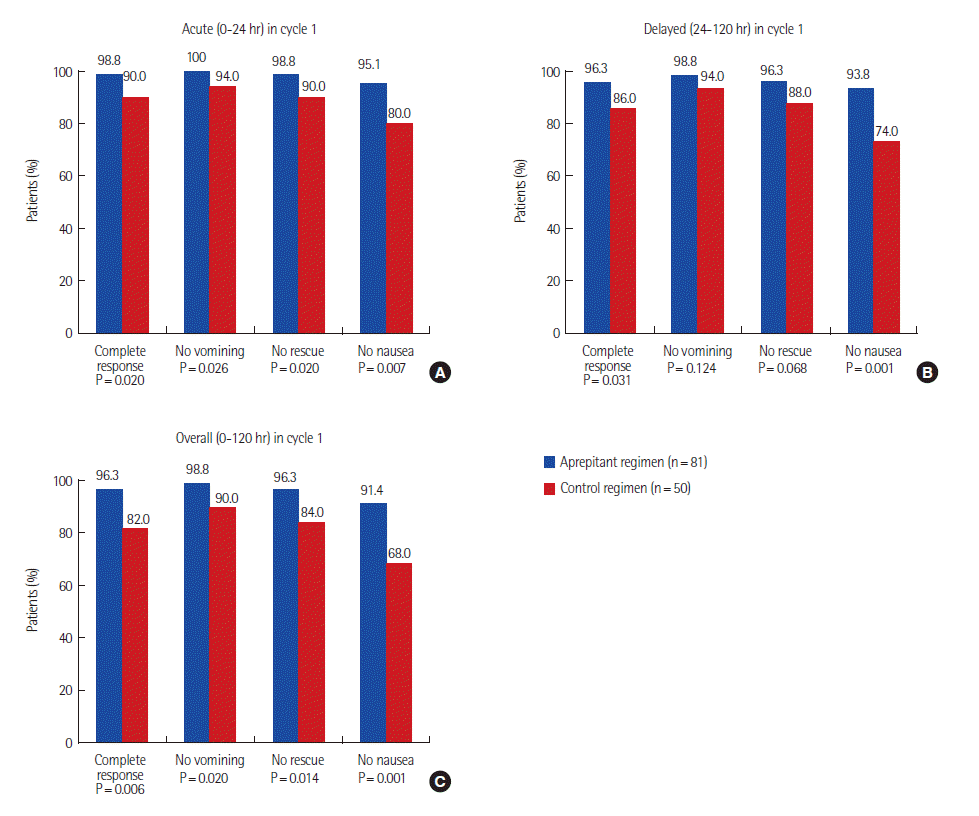
Fig. 1 shows the proportions of patients in each treatment group who had complete response (CR) acute, delayed and overall phases.The efficacy end point was CR, which is defined as no emetic episodes and no use of rescue medication during the 5 days (120 hours) after initiation of chemotherapy [2].
CR rate was significantly higher in the aprepitant group in the acute phase (98.8% vs. 90.0%, P=0.02) and the delayed phase (96.3% vs. 86.0%, P=0.031). Also in the overall phase, significantly more patients in the aprepitant group reported no vomiting compared to those in the control group (96.3% vs. 82.0%, P=0.006).
Kaplan-Meier curves for the time to the first vomiting, regardless of a use of rescue therapy, in the first 120 hours after chemotherapy are shown in Fig. 2. It depict the cumulative percentage of patients who remained vomiting-free since the initiation of chemotherapy and show that the time to first vomiting was longer in patients in the aprepitant group compared with the control group (P< 0.001).
Tolerability
Table 2 summarizes adverse events reported after treatment. The overall incidence of adverse events was similar between the two groups (30% vs. 26%). Although two patients on the aprepitant regimen, discontinued treatment due to neutropenia and regarded as National Cancer Institute common toxicity criteria grade 4, but these events were neither fetal nor drug-related [6].
DISCUSSION
5HT3-receptor antagonist have been recommended for several years, often in combination with corticosteroids, as the agents of first choice for control of CINV [7]. Despite these existing preventative measures, CINV remains a major adverse effect of cancer chemotherapy. Healthcare providers often underestimate the incidence of CINV after MEC. The majority of physicians and nurses had quite accurate perceptions of the control of acute emesis, but overestimated the control of delayed nausea and emesis after MEC [8]. The combination of 5HT3-receptor antagonist with dexamethasone can protect 90% of patients from acute emesis, but these drugs given alone or in combination protect only 40% to 60% of patients from delayed emesis [9]. More effective antiemetic treatments are still needed [10,11]. This has led to the development of a new class of antiemetic agents, such as aprepitant, an antagonist of the NK-1 receptor.
Warr et al. [12] reported that the addition of aprepitant to 5HT3-receptor antagonist and dexametahsone in cisplantin-based chemotherapy significantly reduced acute and delayed emesis. Grunberg et al. [13] described that a single-day three-drug antiemetic regimen is feasible and effective for protection against both acute and delayed vomiting after MEC. As shown in the above theses, several researchers demonstrated that aprepitant-containing antiemetic regimen in patients with a variety of cancer types who were treated with MEC have shown greater efficacy than the control regimen. As s a result, a three-drug regimen including 5HT3-receptor antagonist, dexamethasone, and aprepitant has been incorporated into the Multinational Association of Supportive Care in Cancer (MASCC), European Society for Medical Oncology (ESMO), American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) guidelines for the treatment of patients receiving selected MEC regimens [1,14-16]. That is, aprepitant should be added to dexamethasone and a 5HT3-receptor antagonist for select patients receiving certain moderate emetic risk chemotherapy (i.e., carboplatin, cisplatin, doxorubicin, epirubicin, ifosfamide, irinotecan or methotrexate), because these agents are more emetogenic than other moderately emetogenic agents [16,17].
Treating CINV is important, but to prevent is more important. In fact, prevention is the goal of management of CINV according to ASCO commendation in 2011; for patients who experience anticipatory nausea and vomiting, the use of the most active antiemetic regimens appropriate for the chemotherapy being administered to prevent acute or delayed emesis is suggested. Such regimens should be used with initial chemotherapy, rather than assessing the patient’s emetic response with less effective treatment [18]. Hesketh et al. [19] reported the combination of aprepitant, dexamethasone and palonosetron prevented emesis more than 90% of breast cancer patients receiving their initial cycle of doxorubicin and cyclophosphamide. Warr et al. [2] demonstrated the addition of aprepitant to an antiemetic regimen of ondansetron and dexamethasone resulted in significantly better prevention of CINV than ondansetron and dexamethasone alone in patients with breast cancer after initial cycle of MEC. A similar observation was found in the present study. The addition of aprepitant to the antiemetic regimen of ondansetron and dexamethasone was superior to ondansetron and dexamethasone alone, resulting in greater proportions of patients reporting no vomiting and complete response in the first cycle of MEC.
Aprepitant is a moderated inhibitor of cytochrome P 450 enzyme 3A4 (CYP3A4) and inducer of cytochrome P 2C9 (CYP2C9). Thus aprepitant can alter the metabolism of certain drugs and change their plasma concentrations [14]. Patients should not take aprepitant with pimozide, terfenadine, astemizole, cisapride; these combinations are contraindicated, since inhibition of CYP3A4 by aprepitant could result in elevated plasma concentrations of these drugs, potentially causing serious or life-threatening reactions [20]. Coadministration of aprepitant with warfarin, a kind of CYP2C9 substrate, may result in a clinically significant decrease in international normalized ratio of prothrombin time. The most common side effects of aprepitant are tiredness, nausea, hiccups, constipation, diarrhea, loss of appetite, headache, and hair loss. Clinical adverse experiences for the CINV regimen in conjunction with moderately emetogenic chemotherapy (incidence>10%) are: alopecia, anorexia, asthenia/fatigue, constipation, diarrhea, headache, hiccups, nausea [20]. In our study, aprepitant was generally well tolerated, with an overall pattern of clinical and laboratory adverse events similar to that of the control group. There were no significant differences in adverse events between the group of patients who received the aprepitant regimen and control regimen.
Previous studies of the effect of aprepitant in the first cycle of MEC were almost about the doxorubicin and cyclophosphamide regimen and data are sparse regarding other MEC agents. Korean medical insurance does not cover the aprepitant for the use of antiemetic drug in the first cycle of MEC yet. We guess that this study may provide some support for the effect of aprepitant in the first cycle MEC and justification for the covering by the medical insurance. However the limitation of the study is the relatively small sample size. Therefore, further well-designed clinical trials are required in the future.
In conclusion, aprepitant in combination with 5HT3-receptor antagonist, dexamethasone significantly improved CINV in the initial cycle. We suggest that aprepitant would be necessary from the first cycle as a standard antiemetic agent for colon cancer patients receiving MEC. We also expect the expansion of eligibility of aprepitant to medical insurance.