Unusual mesentery metastasis of differentiated thyroid cancer: a case report
Article information
Abstract
Distant metastases of well-differentiated thyroid cancers (WDTCs) to bone and lungs are well known, while intra-abdominal, mesenteric metastases are very rare. Herein, we report a case of intra-abdominal, mesenteric metastasis of WDTC. A 62-year-old man underwent thyroid lobectomy for follicular thyroid cancer. One year later, lung metastasis was observed. The patient simultaneously underwent lung wedge resection and complete thyroidectomy. Eleven years later, serum thyroglobulin level was elevated. On the work-up study, a metastatic lesion in the lungs and a mass in the mesentery were identified. Two lesions of the lung and mesentery were surgically resected. The mass in the mesentery was pathologically diagnosed as metastatic WDTC.
INTRODUCTION
Well-differentiated thyroid cancers (WDTCs) originating from follicles, including papillary, follicular, and Hurthle-cell subtypes, account for 90% to 95% of thyroid cancers [1]. Of patients with WDTC, distant metastasis occurs in 4% to 15% [2,3]. Except for lymph nodes, the most common metastatic sites are the lungs, bones, and brain [4]. Other than the lungs and bones, distal metastasis of WDTCs is less than 5% [5]. Despite this low probability, intra-abdominal, mesenteric metastasis is very rare. Herein, we report a case of intra-abdominal mesenteric metastasis of WDTC. This report was approved by the Institutional Review Board of Kosin University Gospel Hospital (IRB 2023-06-025). The informed consent was waived.
CASE REPORT
A 62-year-old man was admitted to the surgery department at the hospital due to a mesentery mass observed on positron emission tomography/computed tomography (PET/CT) imaging during a work-up for suspected metastatic follicular thyroid cancer.
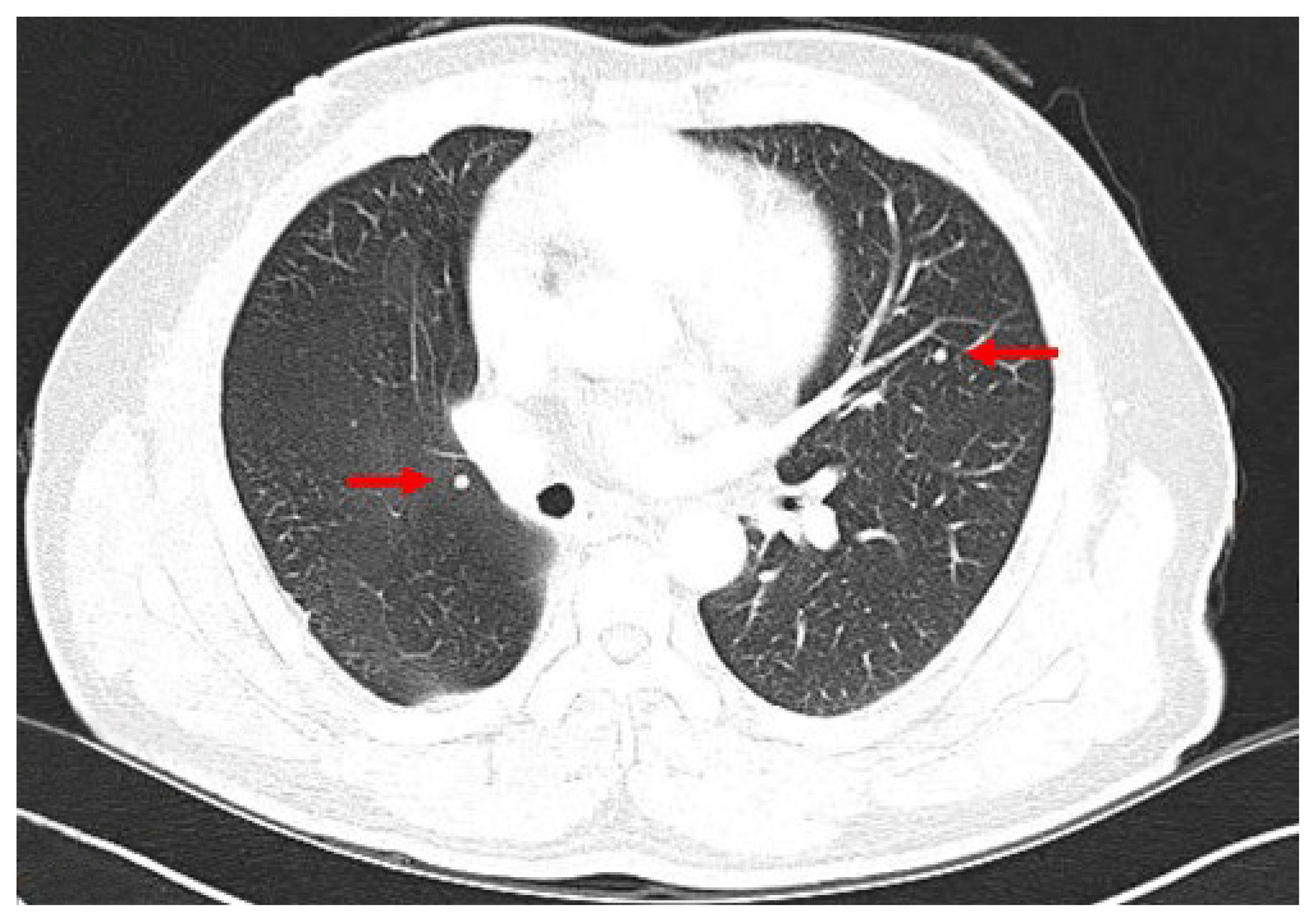
Approximately 11 years prior, the patient had undergone surgery involving a thyroid lobectomy for the mass on the right lobe. Preoperative diagnosis was follicular neoplasm, and the mass was confirmed as follicular cancer with minimally invasive capsular invasion and a vascular invasion focus. At that time, lung, rib, and thyroid masses were observed, and the patient underwent video-assisted thoracoscopic surgery (VATS) lobectomy and rib tumor resection for each. The pathologic results were adenocarcinoma for the lung mass and chondrosarcoma for the rib tumor. About 1 year later on follow-up chest CT, multiple subcentimeter, round, solid nodules were newly discovered in both lungs, and the size of the existing nodules had increased (Fig. 1). A second VATS lobectomy was performed, revealing metastatic follicular cancer. The patient subsequently underwent a complete thyroidectomy with central compartment neck dissection, and its pathologic result was nodular hyperplasia. This was surprising considering the biopsy result of adenocarcinoma at the time of the first VATS lobectomy.
The patient was lost to postoperative follow-up, but he reported two cycles of high-dose radioactive iodine (RAI) treatment and continued follow-up at a local medical center. When the serum thyroglobulin (Tg) level was elevated to 13.94 ng/mL, a tertiary hospital visit was recommended. When the patient visited this hospital, the serum Tg level had increased to 32.50 ng/mL and the serum anti-Tg level was less than 15.0 U/mL. During the work-up for surgery, neck CT revealed focal small enhancing lesions on the left side of the operation bed that were suspicious of remnant thyroid tissue. A small enhancing nodule in the left lower lobe was suspicious of a metastatic nodule on chest CT. Abdominal CT and PET revealed a 3.2 cm, well-defined enhancing mass in the lower upper quadrant that required investigation to rule out a carcinoid tumor, mesenteric fibroma, accessory spleen, etc. (Fig. 2). The tissue of the left lower lobe wedge resection of the lung and the mass on the mesentery, which turned out to a lymph node, was removed with the adjacent small bowel segment. The mass on the mesentery was approximately 4 cm in size (Fig. 3). Its pathologic result was metastatic follicular thyroid cancer based on the findings of less than two mitoses per high power field, no evidence of tumor necrosis, and less than 3% Ki-67 index (Figs. 4, 5). The patient recovered well without acute postoperative complications. The patient underwent a third additional high-dose RAI treatment when the serum Tg and anti-Tg levels were 6.38 ng/mL and 16.6 U/mL, respectively. After 6 months, the serum Tg and anti-Tg levels were 8.22 ng/mL and 1.3 U/mL, respectively.
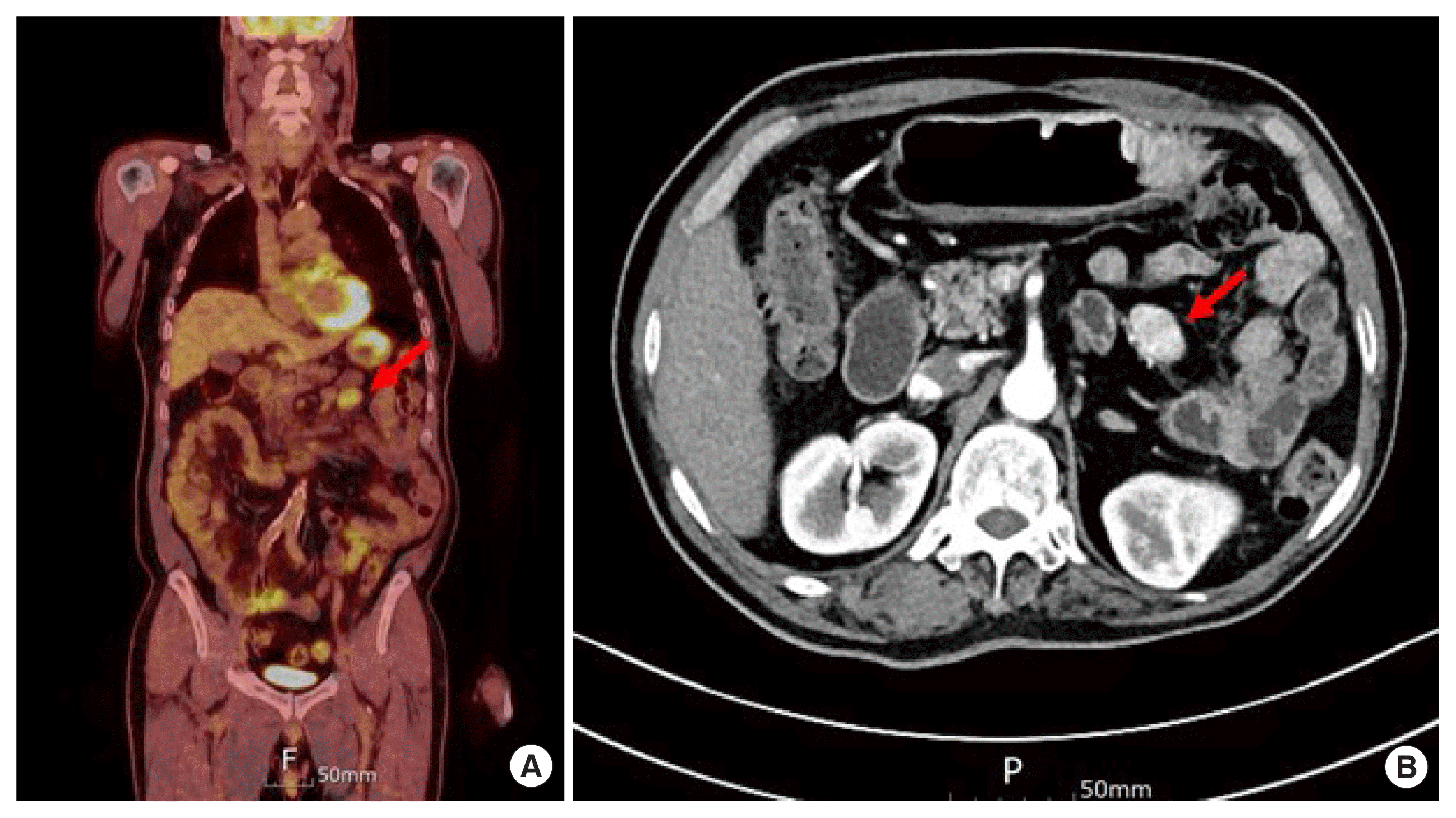
(A) Positron emission tomography/computed tomography (PET/CT) showed increased uptake in mesentery near to Treitz ligament (arrow). (B) CT showed the mass (arrow).

Metastatic follicular thyroid carcinoma (3.9×2.5×2.2 cm) on mesentery. Grossly, the mass was a lump of grayish-brown tissue. The cut surface of the mass is gray, dark brown, smooth, and glistening with hemorrhage.
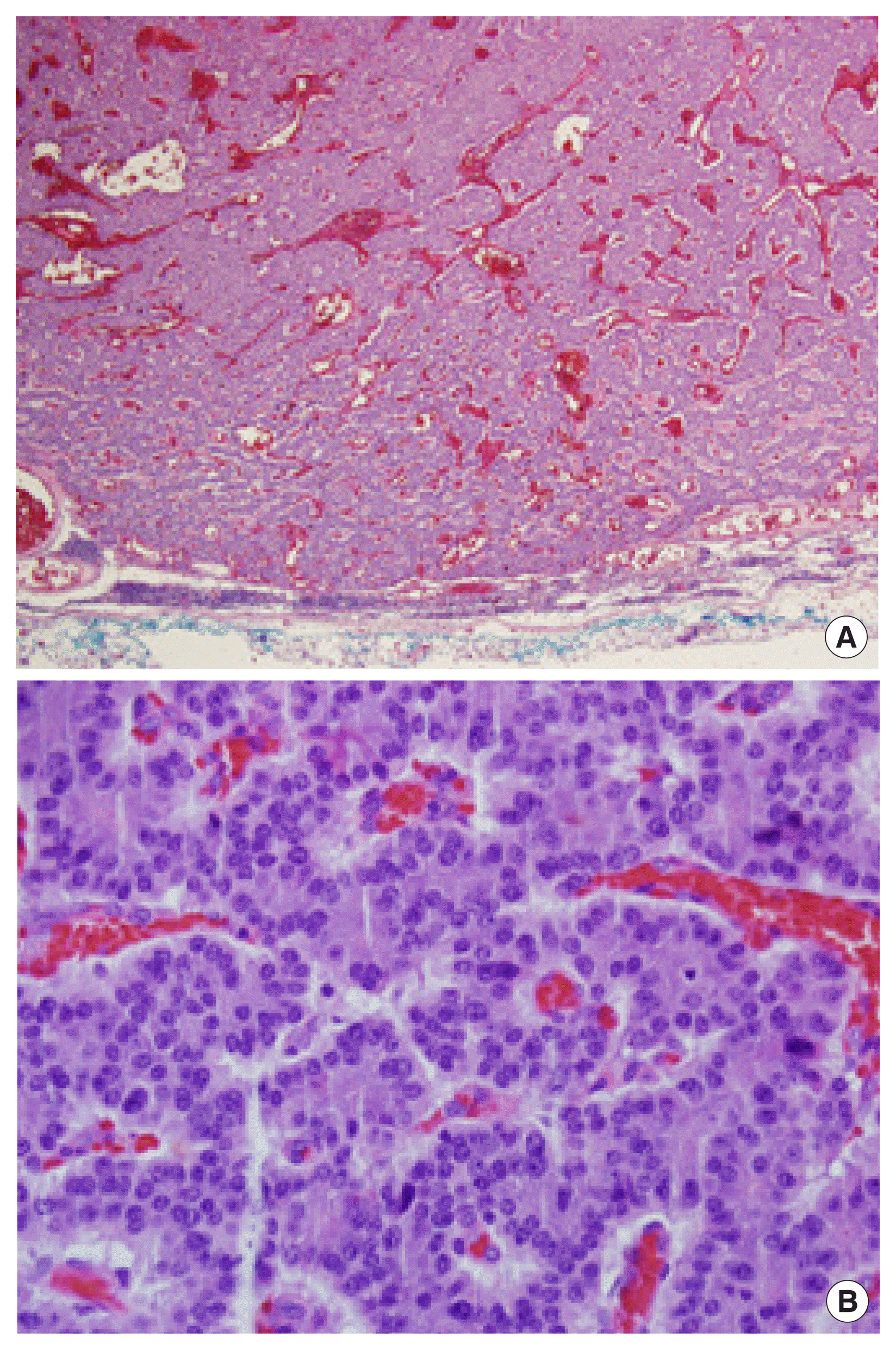
The neoplastic cells are arranged in follicular, solid, or trabecular patterns. (A) Hematoxylin and eosin (H&E) stain, ×40. (B) H&E stain, ×200. There were two mitoses per high power field. Tumor necrosis was not identified.
DISCUSSION
Unusual metastases of WDTC have been described in the literature to involve the brain, parotid gland, pharynx, breast, skeletal muscle, and intra-abdominal organs. In the intra-abdominal organs, metastases to the liver, pancreas, kidney, adrenal gland, and ovary were rare [5–10].
Distant metastasis of WDTC to the mesentery is very rare. Only six or seven cases, under the notation “gastrointestinal” on a figure in a systematic review [5], have been identified, including one case of metastatic follicular thyroid cancer. Generally, follicular thyroid cancer metastasizes hematogenously to visceral organs or bones. Therefore, it seems to make sense that the ratio of follicular to papillary thyroid cancer is higher in some well-vascularized organs. Still, the organ tropism of thyroid cancer is very complex and not well understood.
Serum Tg is a reliable factor to detect metastasis or recurrence because its half-life is approximately 30 hours after a total thyroidectomy [11]. After this procedure, no metastasis is noted when the thyroid stimulating hormone (TSH)-suppressed Tg concentration is less than 2 ng/mL and TSH-stimulated Tg concentration is less than 5 ng/mL. Generally, a TSH-suppressed Tg level of 2 ng/mL or above strongly suggests persistent or recurrent cancer requiring additional evaluation.
In addition, anti-Tg antibodies are detectable in 10% of the general population and 20% of thyroid cancer patients, and neck ultrasound and RAI scan can be helpful tools to evaluate recurrence or metastasis. A fluorodeoxyglucose-PET scan can be used to localize metastatic sites.
Interestingly, in this case, it was determined later on follow-up chest CT (not abdominal CT) that the metastatic lesion on the mesentery had gradually increased in size with undetectable serum Tg level. In other words, the serum Tg level did not increase despite the preexisting metastatic follicular thyroid cancer on the mesentery.
It is not known whether the increase in serum Tg level was due to the newly developed secondary metastatic lesions in the lungs or to growth of the metastatic mass of the mesentery to a certain size. The primary metastatic lung lesion (0.3×0.5×0.4 cm) was detected on CT but not by increased serum Tg level.
However, it is questionable whether this patient’s treatment plan or prognosis would have changed notably if this mesentery metastatic lesion had been detected in previous chest CT without an elevation of serum Tg level.
Among many modulating processes in the oncogenesis of thyroid cancer, potentially unregulated processes have been studied and presented to account for metastasis of thyroid cancers. Some of them explain the propagation of thyroid cancer out of the cervical area [5]. Unfortunately, the explanation of metastasis to rare organs remains unknown.
Given that the 10-year survival rate of primary WDTCs is 80% to 95% [7] but decreases to 30%–50% in the presence of distant metastasis [5], studies on the relationships between the characteristics of distant metastasis and prognosis are needed. Careful monitoring of WDTC metastasis using various methods such as serum Tg and imaging studies is required. In particular, unusual metastasis such as mesentery metastasis must be considered.
Notes
No potential conflict of interest relevant to this article was reported.
FUNDING
None.