Mucinous carcinoma is a predictive factor for the risk of open conversion from laparoscopic colectomy in colorectal cancer
Article information
Abstract
Purpose
Although laparoscopic surgery is widely accepted in the treatment of colorectal cancer, conversion to open surgery is associated with the rate of unfavorable outcomes. The aim of this study was to determine the factors associated with open conversion from laparoscopic surgery for colorectal cancer.
Methods
A total of 3,002 patients who underwent laparoscopic colectomy as an initial plan for the treatment of colorectal cancer located from the sigmoid colon to the rectum were retrospectively evaluated between January 2009 and December 2018 at Samsung Medical Center in Korea. Risk factors significantly associated with open conversion were determined using univariate and multivariate regression models.
Results
Among the 3,002 patients, open conversion was performed in 120 patients (4%). Age >60 years (adjusted odds ratio [AOR], 2.370), preoperative bowel obstruction (AOR, 2.348), clinical T4 stage (AOR, 2.201), and serum carcinoembryonic antigen level >5 ng/mL (AOR, 2.289) were significantly associated with open conversion. Moreover, mucinous carcinoma was a significantly more frequent histopathologic type than adenocarcinoma (10.0% vs. 3.2%, P<0.001) in the open conversion group with an AOR of 2.549 (confidence interval, 1.259–5.159; P=0.009).
Conclusion
The present study presented a novel finding, i.e. mucinous carcinoma as the histopathologic type could be an independent predictive factor for conversion from laparoscopic colectomy to open surgery. Identifying patients with mucinous carcinoma will help stratify the risk of open conversion preoperatively.
INTRODUCTION
Laparoscopic colorectal surgery is widely performed in the resection of colorectal cancer owing to its lower complication rate, lower rate of perioperative morbidity, and shorter hospital stay compared with open surgery [1,2]. However, there have been concerns about the risk of conversion to open surgery during laparoscopic colorectal surgery [3].
Several studies have reported higher rates of postoperative morbidity from open conversion [4,5]. The risk factors associated with open conversion have been identified, including body mass index (BMI), American Society of Anesthesiologists (ASA) physical status classification, left-sided and low anterior resection, clinical T4 stage, acute surgery, metastatic setting, sex, age, and hospital volume [3,6,7]. However, whether the histologic findings of colorectal cancer affect the conversion of laparoscopic colorectal surgery to open surgery is not well established.
In colorectal cancer, almost 90% of cases are adenocarcinoma in histologic tumor subtypes. Among the other subtypes, mucinous carcinoma is observed in approximately 4%–19% of cases and more commonly found in the proximal colon [8]. Nevertheless, when mucinous carcinoma presents in the rectum, it behaves more aggressively [9]. A study suggests that the reason for aggressiveness when located in the rectum may be due to the anatomical effect of the pelvic cavity [10].
The purpose of the present study was to evaluate the factors associated with the risk of open conversion with respect to the pathologic types of colorectal cancers, especially those located in the sigmoid colon and rectum.
METHODS
Patient inclusion criteria and data collection
Patients who were initially planned to undergo laparoscopic surgery between January 2009 and December 2018 at Samsung Medical Center for colorectal cancer below the descending colon, including the sigmoid colon, rectosigmoid junction, and rectum, were retrospectively evaluated. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB No. 2019-10-156) before data collection began. Data on patients’ demographics, underlying diseases, prior abdominal surgery (PAS) history, preoperative bowel obstruction, carcinoembryonic antigen (CEA) serum levels measured before and after surgery, neoadjuvant concurrent chemoradiotherapy (CCRT) for rectal cancer, pathologic diagnosis of surgical specimens, pathologic stage of cancer, and cases of open conversion were collected from medical records. PAS was classified as major and minor PAS. Major PAS includes cases of abdominal surgery involving more than one abdominal quadrant. Minor PAS was defined as abdominal surgery involving one abdominal quadrant such as laparoscopic surgery with cholecystectomy and appendectomy, and benign gynecologic surgery [11]. Mucinous carcinoma is defined as a tumor with >50% of extracellular mucin component confirmed through microscopic inspection [12,13]. Patients with lymphoma, familial adenomatous polyposis or hereditary nonpolyposis colorectal cancer, and rectal neuroendocrine tumor, as well as those who had surgical resection for recurrent colorectal cancer were excluded. The patients were categorized into two groups: a group in which conversion of laparoscopic surgery to open surgery was needed, and another group in which laparoscopic surgery was completed without conversion to open surgery.
Determination of the need for conversion of laparoscopic surgery to open surgery
Conversion is an intraoperative switch from a laparoscopic approach to an open abdominal approach. There are two subtypes of conversion: strategic conversion and reactive conversion. Strategic conversion means a standard laparotomy which is decided directly after the assessment of the feasibility of completing the procedure laparoscopically, the operative difficulty, or logistic consideration. It was not considered as a conversion when laparotomy chosen after a diagnostic laparoscopy (i.e., to assess the curability of the disease). Reactive conversion is the need for a laparotomy because of complications or operative difficulties after a considerable amount of dissection [14]. In this study, patients who met the criteria of one of the two subtypes were included in the conversion group. The reasons for conversion were categorized as follows: patient’s physical environment, intraoperative bleeding, severe adhesion caused by a previous abdominal operation or with unknown cause, cancer invasion to adjacent organs, perforation, obstruction, advanced N stage, positive distal resection margin, problems related to the anastomosis site, peritoneal seeding, and desaturation after pneumoperitoneum. Conversion owing to the patient’s physical environment was defined as when conversion was needed because of the patient’s narrow pelvic cavity, anatomical variations, or underlying diseases such as liver cirrhosis or a huge benign ovarian mass. Open conversion owing to advanced N stage was based on the presence of suspicious metastatic lymph nodes beyond the surgical plane. Anastomosis-related problems that caused conversion from laparoscopy to open surgery included bowel color change or devascularization of the anastomosis site after the completion of the anastomosis procedure.
Statistical analysis
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. The normality of data was tested using the Kolmogorov-Smirnov method. The independent t-test was used to evaluate statistically significant differences between mean values of continuous variables. The chi-square test was performed to compare categorical variables. Values of P<0.05 were considered statistically significant. Variables potentially related to the risk of open conversion with P<0.05 on univariable analysis were included in multivariate analysis.
RESULTS
A total of 3,002 patients diagnosed with sigmoid or rectosigmoid junction or rectal cancer were initially planned to undergo laparoscopic colorectal cancer surgery. Of these patients, 2,882 (96%) underwent laparoscopic completion colectomy for colorectal cancer and 120 underwent conversion to open colorectal surgery (conversion rate, 4%). Among the 120 patients, 65 (54.1%) were strategic conversion and 55 (45.9%) were reactive conversion.
The demographic features of patients and preoperative clinical data including the location of the cancer, major or minor PAS, BMI, CCRT, and preoperative serum levels of CEA and carbohydrate antigen 19-9 (CA19-9) are summarized in Table 1. The median age was 66 and 60 years in the conversion group and non-conversion group, respectively, and was significantly higher in the conversion group (mean±standard deviation: 66.68±13.4 years vs. 60.28±11.66 years, P<0.001). There were no differences in sex (P=0.771) or cancer location between the two groups (Table 1).
PAS (P=0.617) showed no difference in the conversion group and the non-conversion group. In addition, major PAS and minor PAS had no significant differences (P=0.138 and P=0.824, respectively). Preoperative serum level of CA19-9 (P=0.870), preoperative CCRT (P=0.87), and underlying diseases including hypertension (P=0.366) and diabetes mellitus (P=0.209) appeared to have no significant differences between the two groups.
On the other hand, the presence of preoperative obstruction (37.5% vs. 13.1%, P<0.001) and clinical T4 stage (29.2% vs. 10.2%, P<0.001) were more frequently observed in the open conversion group than in the laparoscopic completion group. Additionally, 27 (22.5%) patients in the open conversion group and 421 (14.6%) patients in the laparoscopic completion group had N2 stage, with a statistically significant difference (P=0.019). The preoperative serum level of CEA tended to be more elevated in patients who underwent conversion from laparoscopic to open surgery than in those who underwent laparoscopic completion (P=0.005). With respect to the histologic cancer subtypes (adenocarcinoma or mucinous carcinoma), there were major differences between the two groups. Mucinous carcinoma was diagnosed in 10 patients (10%) in the open conversion group compared with 92 patients (3.2%) in the laparoscopic completion group (P<0.001), as shown in Table 1.
The reasons for conversion are summarized in Table 2. Cancer invasion to adjacent organs was the most frequent reason for conversion (43.3%), followed by adhesion (12.5%), intraoperative bleeding (10.8%), and obstruction (10%). Further, there were other factors for open conversion during laparoscopic surgery, including peritoneal seeding during laparoscopic exploration in seven patients (5.8%), a narrow pelvis or a friable and easy-to-bleed tissue due to underlying liver cirrhosis, or a fatty mesentery, or a huge ovarian mass that prevented the laparoscopic procedure in six patients (5%), a bowel color change or a devascularization of the anastomosis site after anastomosis in four patients (4.3%), and suspicious metastatic lymph nodes found beyond the surgical field (e.g., para-aortic or pelvic lesions) in three patients (2.5%) (Table 2).
Univariate and multivariate analyses were performed to evaluate the risk factors for conversion from laparoscopic to open colectomy (Table 3). Univariate analysis showed that age ≥60 years (odds ratio [OR], 2.407; 95% confidence interval [CI], 1.631–3.551; P<0.001), preoperative CEA serum level >5.0 ng/mL (OR, 3.423; 95% CI, 2.332–5.025; P<0.001), clinical T4 stage (OR, 3.637; 95% CI, 2.409–5.490; P<0.001), N2 stage (OR, 1.695; 95% CI, 1.091–2.634; P=0.019), bowel obstruction (OR, 3.987; 95% CI, 2.712–5.860; P<0.001), and mucinous carcinoma as the histologic type (OR, 3.370; 95% CI, 1.792–6.336; P<0.001) had higher odds ratios in the open conversion group than in the laparoscopic completion group. Preoperative CCRT did not seem to increase the risk for open conversion (OR, 0.775; 95% CI, 0.454–1.324; P=0.870), as it showed no significant difference between the two groups (Table 3).
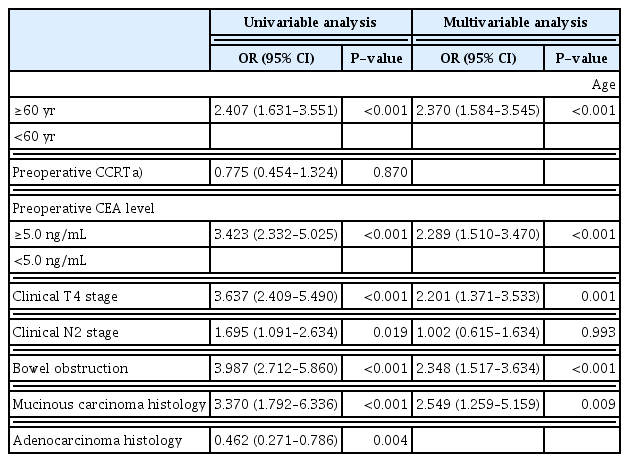
Risk factors of open conversion from laparoscopic surgery in univariable and multivariable analyses of the cohort (n=3,002)
The risk of conversion was further analyzed with respect to the associations between variables (Table 3). Among them, the risk of conversion was the highest with the mucinous carcinoma histology, with an adjusted odds ratio (AOR) of 2.549 (95% CI, 1.259–5.159; P=0.009). Moreover, the odds of open conversion were higher with bowel obstruction (AOR, 2.348; 95% CI, 1.517–3.634; P<0.001), preoperative CEA serum level >5.0 ng/mL (AOR, 2.289; 95% CI, 1.510–3.470; P<0.001), clinical T4 stage (AOR, 2.201; 95% CI, 1.371–3.533; P<0.001), and age (AOR, 2.370; 95% CI, 1.584–3.545; P<0.001). However, clinical N2 stage was not associated with the risk of conversion.
DISCUSSION
In 2016, Stormark et al. [7] studied a large national patient cohort and confirmed the safety of laparoscopic colectomy and its similar long-term survival to that of open surgery. A recent study showed that the use of laparoscopic colectomy has increased to >80% and the conversion rate has decreased from 12% to 8% in colorectal cancer between 2011 and 2015. The decrease in conversion was significantly related to the increased laparoscopic volume in hospitals [15]. We observed that the conversion rate for sigmoid colon, rectosigmoid colon, and rectal cancer was 4.0% between 2009 and 2018.
We found that the most frequent reason for conversion was invasion of cancer to adjacent tissues or organs, which accounts for nearly 50%. Allaix et al. [16] reviewed all literature on cases of open conversion during laparoscopic resection for both colon cancer and rectal cancer for all years up to March 2016, and the most frequent reasons for conversion were also cancer-related factors. The other causes they reported were adhesion, obesity, or anatomic-related factors, which were similar to the results of our study.
Although several previous studies reported that male sex and obesity increased the risk of conversion [1,4], sex and BMI were not associated with conversion in this study. Some patients underwent conversion from laparoscopic to open surgery because of adhesions from previous abdominal operations. Kim et al. [11] found that major PAS and minor PAS presented different results. Patients with a history of major PAS showed a higher conversion rate than those with no PAS. In contrast, patients with a history of minor PAS and those with no PAS showed no difference in conversion rate. In our results, major PAS had no statistically significant difference between the conversion group and the laparoscopic completion group.
There have been some efforts to clarify the relationship between neoadjuvant chemoradiotherapy and technical difficulties in laparoscopic surgery for rectal cancer [17,18]. A study examined the effect of preoperative chemoradiotherapy in laparoscopic surgery for rectal cancer, and found no difference in conversion rate between the preoperative chemoradiotherapy group and the surgery only group (18.8% and 15.4%, respectively; P=0.392) [19]. Correspondingly, we observed that preoperative chemoradiotherapy had no effect on the conversion from laparoscopic to open surgery. Because some studies found that high ASA scores increased the risk of conversion [3,6], we also investigated patients with hypertension or diabetes mellitus. However, hypertension and diabetes mellitus had no individual effect on the conversion rate.
After multivariate analyses, we found five factors that could be independent predictors of conversion from laparoscopic to open surgery. Although higher age was not a risk factor for open conversion in previous studies [6,7], patients aged >60 years had an almost 2-fold higher conversion risk in this study. Stormark et al. [7] and Tekkis et al. [3] already confirmed that higher T and N stages were associated with conversion from laparoscopic to open surgery. We also identified that patients with clinical T4 stage had a higher risk of conversion (AOR, 2.2). However, clinical N2 stage was not an individual risk factor in our study. Preoperative obstruction was also a strong risk factor that significantly affected the conversion risk (AOR, 2.3) in our data. Several studies had reported that emergent colorectal resection had a higher conversion rate than elective colorectal resection [7,20].
There have been no reports on the relationship between preoperative serum CEA and CA19-9 levels and the conversion risk in laparoscopic colorectal resection for colorectal cancer. Comparing the laparoscopic completion group and the open conversion group, we confirmed that preoperative serum CEA serum level tended to be much higher in the open conversion group. A >5.0 ng/mL serum CEA level was an independent risk factor with an AOR of 2.3. Although higher serum levels of both CEA and CA19-9 are related to the severity of colorectal cancer [21], the level of serum CA19-9 was not a risk factor for conversion in our findings.
In this study, mucinous carcinoma was diagnosed in 12 patients (10%) in the open conversion group and in 92 patients (3.2%) in the laparoscopic completion group (P<0.001). In addition, we observed that mucinous carcinoma as the histopathologic type was an independent risk factor with an AOR of 2.5. There have been no studies on the association between the histologic type of cancer and the risk of conversion. Mucinous carcinoma is diagnosed in 4% to 15% of all patients with colorectal cancer [22]. This histologic type is much rarer in the rectum [23] and more frequently found in the proximal colon [24]. Mucinous carcinoma is diagnosed if the tumor cells produce mucin that aggregates into pools or lakes, occupying >50% of the tumor [25]. As the definition of mucinous carcinoma has varied according to the amount of mucus produced by neoplastic cells within the rectum over the years, mucinous tumors of the rectum are less well understood than non-mucinous tumors [10]. Several studies have observed that mucin has a pressure effect that causes dissemination of cancer cells into the peritoneal cavity [26–28]. Moreover, compared with adenocarcinoma, mucinous carcinoma is more frequently found at a higher disease stage at presentation [9,29]. Hyngstrom et al. [9] found that mucinous cancer located in the rectum showed worse survival outcomes with a hazard ratio (HR) of death of 1.22, but not for those located in the colon (HR, 1.03). Chand et al. [10] explained that mucinous carcinoma in the rectum has a more aggressive behavior than mucinous carcinoma in the colon because of the anatomical location and the immediate effect of the narrow pelvic cavity. In the present study, the association between the mucinous histologic type and open conversion in low colorectal cancer can be explained by the unique clinicopathologic behavior of mucin. Table 4 shows the clinical reason for open conversion in 12 patients in whom the histologic type of cancer was mucinous carcinoma. All 12 cases were converted to open laparotomy from laparoscopic colectomy due to invasion or adhesion. Identifying patients with mucinous carcinoma in preoperative colonoscopy biopsy or those suspicious of having mucinous carcinoma in preoperative magnetic resonance imaging will aid the prediction of the possibility of open conversion by understanding the unique features of mucinous carcinoma.

The details for the patients with mucinous carcinoma who were converted open colectomy from laparoscopic colectomy
The predictive factors we investigated would allow surgeons to prepare for cases of open conversion by stratifying the conversion risk. However, it remains difficult to draw a proper conclusion owing to the inherent limitations of a retrospective study and the small sample size in subgroup analysis. To strengthen our results, further evaluations of mucinous carcinoma located in proximal colon and multicenter studies will be required. In addition, further studies on short- and long-term outcomes comparing the laparoscopic completion group and the open conversion group of patients with mucinous carcinoma would provide more evidence for determining the treatment plan.
In conclusion, this study demonstrated that age >60 years, clinical T4 stage, bowel obstruction by the cancer, CEA serum level >5.0 ng/mL, and mucinous carcinoma as the histologic type could be independent predictors of conversion from laparoscopic to open colectomy in colorectal cancer.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.


