결장암 환자에서 보조적 항암화학요법 중 삶의 질 변화
Quality of life changes during adjuvant chemotherapy in patients with colon cancer
Article information
Trans Abstract
Purpose:
The survival of advanced colon cancer patients has increased due to the development of surgical techniques and adjuvant chemotherapy. The administration of adjuvant chemotherapy after curative resection is generally accepted as a standard of care. The primary endpoint of chemotherapy should include not only tumor response and survival, but also impact on the quality of life (QoL). We evaluated changes in QoL during adjuvant chemotherapy in patients with colon cancer.
Methods:
Between October 2009 and February 2012, 56 patients with stage II and III colon cancer received the combination adjuvant chemotherapy 5-flurouracil/folinic acid with oxaliplatin (FOLFOX). Patients were asked to complete the QoL questionnaire QLQ-C30 version 3 before and after 6 cycles of adjuvant chemotherapy.
Results:
There was no significant difference in the QoL between the start of chemotherapy and after the completion of 6 cycles. After completion of 6 cycles, global QoL was worse in patients >70 years of age. The functional scale score was low in patients with chemotherapy schedules delayed more than 2 times due to adverse events. Patients with body weight increases greater than 5% scored lower on symptom scales. Interestingly, patients with peripheral neuropathy scored higher on symptom scales.
Conclusion:
QoL changes during adjuvant chemotherapy did not show significant differences. After the sixth chemotherapy, QoL was affected by age, body weight gain, delay of the scheduled chemotherapy, and peripheral neuropathy. Therefore, the proper attitude of physicians focused on reassurance and education of patients is very important during chemotherapy.
INTRODUCTION
The incidence of colorectal cancer in Korea has increased rapidly recently due to changes in environmental factors such as the consumption of a westernized diet. According to 2012 Korean cancer statistics, colon cancer is ranked third after thyroid and stomach cancers, respectively. Five-year relative survival rates in colon cancer have increased 16.8% in recent years from 58.0% in 2000 to 74.8% in 2012 [1]. These changes can be attributed to the early detection of colorectal cancer by colonoscopic screening, the development of adjuvant chemotherapy after surgery, and new surgical techniques.
Treatment modalities of colorectal cancer include surgery, chemotherapy, and radiation therapy. Specifically, adjuvant chemotherapy after curative colon resection is performed in stage II with high risk and stage III. The adjuvant chemotherapy, a foundation of 5-fluorouracil (5-FU) for advanced colon cancer was reported with improvements to the five year disease-free survival and overall survival in the National Surgical Adjuvant Breast and Bowel Project protocol C-03 study [2]. The 5-flurouracil/folinic acid with oxaliplatin (FOLFOX) regimen, composed of oxaliplatin, 5-FU, and leucovorin (LV) demonstrated a significantly improved 3 years disease-free survival in stage II and III colon cancer than that of the same regimen of only 5-FU and LV [3]. Additional follow-up studies of the FOLFOX regimen confirmed a benefit to overall survival [4]. Based on these study results, the FOLFOX regimen has been applied to stage II and III colon cancers. Adverse events such as nausea, vomiting, and leucopenia from the chemotherapeutic agent resulted in deteriorations in quality of life (QoL) [5]. Chemotherapy induced peripheral neuropathy by oxaliplatin is a known typical adverse event associated with decreases in QoL [6]. The primary endpoint of past chemotherapy studies was assessment of treatment effects including tumor response, and survival rate. Recently, the evaluation of QoL has become even important in the care of cancer patients [7-10].
Measurements of QoL were previously important mainly in palliative chemotherapy rather than adjuvant settings [10,11]. The latest investigations of palliative chemotherapy were focused on adverse events, administration methods, drugs before chemotherapy, and the prevention of peripheral neuropathy. QoL is defined in different manners based upon the pluralistic background of a given patient [12]. In order to enhance QoL, physical, emotional, social, and spiritual aspects must be fulfilled.
Interest has recently increased regarding QoL, but research focused on adjuvant chemotherapy is not abundant. Furthermore, most research included various malignances, preconditioning drugs, antiemetics, anticancer regimens with control groups were heterogeneous, and results from these studies are inconsistent [5,10, 11,13]. Many studies regarding QoL in colon cancer patients also included rectal cancer patients. Patients diagnosed with rectal cancer suffer from stoma existence, impairment of defecation or urination, and sexual dysfunction [14,15]. As such, analysis of QoL during adjuvant chemotherapy of colorectal cancer patients should have selection bias in the results. To analyze their QoL, this study includes stage II high risk and stage III patients diagnosed with colon cancer receiving the adjuvant chemotherapy FOLFOX.
METHODS
From October 2009 to February 2012, 56 patients who received the adjuvant chemotherapy FOLFOX after curative resection for colon cancer in Chungbuk Natinal University Hospital Hospital were enrolled in the study. Patients with pathologic results at stage II high risks (pathologic T stage 4, poorly differentiated histology, perineural invasion, lymphovascular invasion, bowel obstruction at presentation, and T3 lesions with localized perforation or close, indeterminate, or positive resection margins) or stage III received chemotherapy within 6 weeks after radical surgery.
Patient information such as age, gender, underlying disease, job, religion, education, and marital status was collected from medical records gathered when patients were admitted. Adverse events were collected during the administration of adjuvant chemotherapy. To score the response levels, this study employed QLQ C30 (version 3.0) developed by European Organization for Research and Treatment of Cancer (EORTC), which was translated and validated in Korean [16]. Questionnaires were composed of three subgroups, global health status, functional scale, and symptom scale. The functional scale includes physical functioning, role functioning, emotional functioning, cognitive functioning, and social functioning. The symptom scale includes fatigue, nausea, vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties. The scale uses a linear transformation to standardize the raw score, so that scores range from 0 to 100; a higher score represents a higher (“better”) level of functioning, or a higher (“worse”) level of symptoms. The health related QoL questionnaire for this study was reviewed when patients in the ward were enrolled in the study, following their review of a consent form describing the contents of the study, and agreed to participate in the study. The questionnaire was examined before chemotherapy and after 6 cycles through one-on-one interviews.
Statistical analysis was performed using PASW software ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Clinical, pathologic, and demographic characteristics were analyzed using the χ2 test (Fisher exact probability test) and the Student t-test or the Wilcoxon ranksum test, depending on the distribution of the variables. QoL scores were described using the mean±standard deviation. We used a paired sample t-test to compare QoL scores before chemotherapy and after the 6 cycles of chemotherapy. All statistical tests were 2-sided. A P-value of less than 0.05 was considered statistically significant.
RESULTS
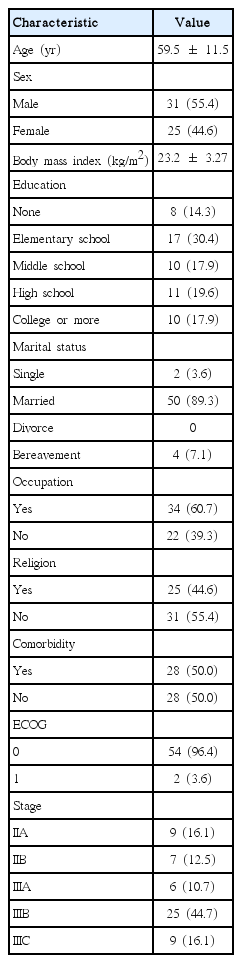
Mean age of patients was 59.5, and there were 7 more male (55.4%) than female patients (Table 1). All patients received more than 6 cycles of chemotherapy with FOLFOX. Most patients’ ECOG activity level ranked in the first grade (n=54, 96.4%). The education attainment level of the plurality of patients (17, 30.4%) was limited to elementary school graduation, 34 patients had jobs (60.7%). Stage IIIB patients represented 44.7% of the patient population.
Nausea was the most frequent adverse effect during the 6 cycles of chemotherapy; 40 patients (71.4%) reported nausea (Table 2). Peripheral neuropathy developed in 31 patients (55.4%) after oxaliplatin administration. One patient reduced the drug dose, and had to stop chemotherapy after the 11th cycle due to severe neuropathy. Neutropenia occurred in 34 patients (60.7%), and one had febrile neutropenia. One patient had an allergic reaction such as fever, sweating, and skin rash within 30 minutes after oxaliplatin administration. This patient was recovered after immediate treatment with anti-histamine (Chlorpheniramine 4 mg) and steroid (Dexamethasone 10 mg) intravenously. Second prevention regimen with a dexamethasone and chlorpheniramine was applied at 30 minutes before chemotherapy start. A single patient refused the chemotherapy, and stopped treatment after the eighth cycle.
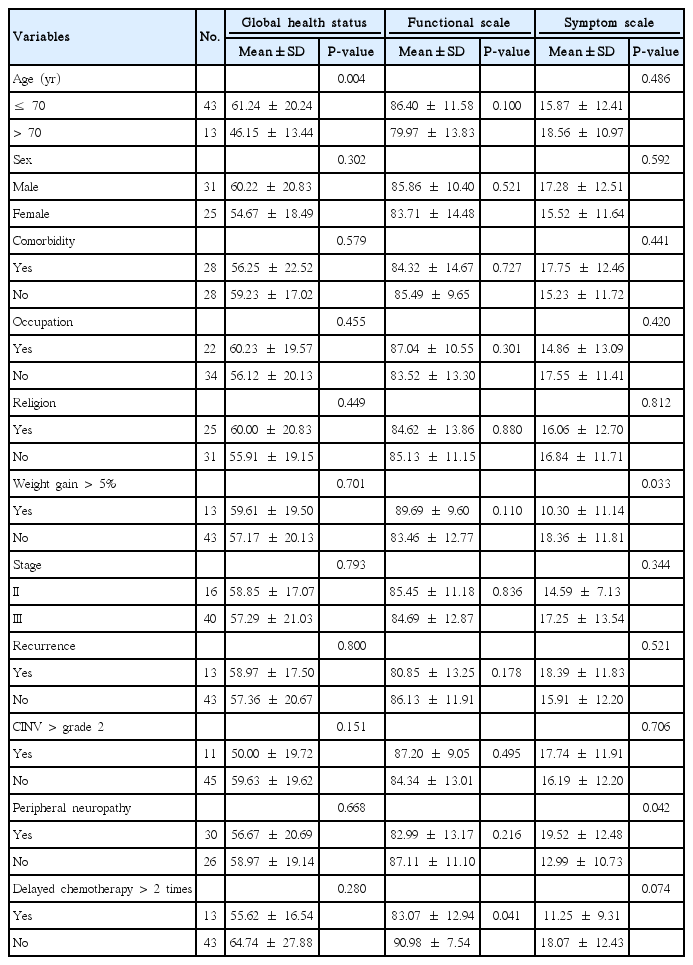
Between the start of chemotherapy and after 6 cycles of FOLFOX, health related QoL was not different (Table 3). Before the start of chemotherapy, global health status scaled at 62.80 and after the sixth cycle, it scaled at 57.74. There was no significant difference statistically between the two time points (P=0.093). Both functional scales were higher than 80.
Two factors impacted QoL during chemotherapy. Patients over 70 years old experienced poorer global health status than younger patients, and when patients gained weight, they had a better QoL (P=0.033). Patients who had oxaliplatin induced peripheral neuropathy, had significantly high symptom scale scores (P=0.042), and if chemotherapy was delayed more than twice due to adverse effects, the functional scale score was low (P=0.041). Job and religion did not affect QoL during chemotherapy (Table 4). Patients with underlying diseases scored low for physical (P=0.010) and social functioning (P=0.013), which are subitems on the functional scale. The highest score of social functioning was found in patients who had no cancer recurrence within 3 years after surgeries (P=0.044). The patient who postponed chemotherapy due to adverse effects, scored significantly lower in role functioning (P=0.021), suffered from nausea/vomiting (P=0.001), and anorexia (P= 0.004). Patients with peripheral neuropathy scored low in emotional (P=0.022) and cognitive functioning (P=0.041). Severe nausea/ vomiting (P=0.002) and diarrhea (P=0.010) troubled them.
DISCUSSION
Radical surgeries and adjuvant chemotherapy have improved the survival of colon cancer, and consequently interests regarding QoL have grown. QoL during chemotherapy has previously been measured mostly in palliative chemotherapy, with research focused on drug selection or administration methods [10,11]. In colon cancer, QoL during adjuvant chemotherapy has not been well studied and evaluated aside from published studies with a heterogeneous group, which included rectal cancer patients. Rectal cancer patients have a different QoL from colon cancer patients [14,15]. In general health status, there is mere distinction between the colon cancer patient and the rectal cancer patient. However, among rectal cancer patients, those with a stoma reported significantly worse QoL than those who did not [17]. Rectal cancer patients undergoing low anterior resection could suffer postoperatively from fecal urgency, incontinence, urinary and sexual dysfunction. Low anterior resection syndrome (LARS) is found in nearly 60% of patients, and is associated with deterioration of QoL in patients [14]. Juul et al. [15] investigated QoL in patients with LARS, which reported serious decreases in general health status, and role/emotional/social functioning. Fatigue and diarrhea were significantly increased in these patients. Drugs introduced, administration methods, and cancer stage also relate to QoL. This current study analyzed changes in QoL, including the factors affected by adjuvant chemotherapy FOLFOX administration, in a homogenous group of colon cancer patients after radical surgeries.
There are well known factors that affect QoL for the patients undergoing chemotherapy. Age, nutritional status, operation, adverse effects, and administration methods are these factors. In the regimen that included oxaliplatin, peripheral neuropathy was the most discouraging factor patients [8,9,14,15]. In particular, QoL before and during palliative chemotherapy is a good survival indicator for those with metastatic colon cancer [18]. Dancey et al. [19] completed a QLQ-C30 survey, and concluded better QoL is associated with improved survival outcomes. Efficace et al. [20] reported the social functioning scale was an independent prognostic factor in patients with advanced colorectal cancer. A study by Earlam et al. [21] of 50 colon cancer patients with liver metastasis, used the Rotterdam symptom checklist, hospital anxiety and depression scales. This study concluded, that diarrhea, anxiety, sleep, and ability to work were prognostic factors for survival. Another cohort study by Maisey et al. [22] with advanced colorectal cancer patients, recommends measurement of QoL before cancer treatment as an important prognostic factor. In this study patients with cancer recurrence within 3 years, did not show a difference in QoL between before, during, and after 6 cycles of adjuvant chemotherapy. Furthermore, adverse effects were analyzed based on patient claims they influenced QoL; these included nausea/vomiting (P=0.722), neutropenia/thrombocytopenia (P=0.971), and paresthesia (P=0.982), yet they did not show a significant difference. Patients with colon cancer have a different concept regarding QoL than the general population. A reframing phenomenon that is changing the perception of positive evaluation of QoL while adapting to cancer treatments has appeared in patients with colon cancer [8,23]. The authors suggest that patients included in the study that had no cancer recurrence within 3–5 months after the sixth cycle of adjuvant chemotherapy would not have significant differences in QoL because of the positive assessment occurring with emergence of the reframing phenomenon.
Age was an important factor influencing QoL during chemotherapy in this study, as patients over 70 years experienced worsening of their general health status. That perception of numerous patients was that further adjuvant therapy meant failure of cancer treatment. This is a recognized issue that has not been solved by the implementation of the adjuvant chemotherapy and it may continue to contribute to the deterioration of QoL. If patients maintained the standard chemotherapy regimen, only chronological age influenced their QoL. However, physicians, patients, and their families often underestimate life expectancy, and tend to neglect opportunities for adjuvant treatments leading to the under-treatment of the elderly. Recent studies suggest patients over 70 years, are influenced more by symptoms of hematologic toxicity than those associated with peripheral neuropathy, infection, and gastrointestinal toxicity [24,25]. This study demonstrates the same result, as age difference was not related to frequency of neutropenia, thrombocytopenia, and nausea/vomiting. Therefore, with proper management targeting a reduction in hematologic toxicities, such as dosage reduction or supportive treatment for adverse effects, QoL for the elderly can be enhanced.
The adjuvant chemotherapy regimen including oxaliplatin, has improved survival of colon cancer patients. However, peripheral neuropathy is a dose-limiting toxicity of chemotherapeutic agents including platinum, vincaalcaloid, taxene that worsens QoL [26]. Approximately 85%–95% of patients suffer from peripheral neuropathy, a result of dose intensity and the cumulative dose. Grade 2 or more of oxaliplatin induced peripheral neuropathy generally occurred in patients who received more than the average cumulative dose of 676–880 mg/m2 [27]. In this study, the average cumulative oxaliplatin dose was 472±24.2 mg/m2, and there was no difference in the cumulative dose associated with the presence of neuropathy. The average dose for 31 (55.4%) of patients was generated from the patient’s peripheral neuropathy, and showed a low rate compared to that in previous studies. We considered a relatively higher rate even though the cumulative dose was less than 676 mg/m2 in all patients. In this study, peripheral neuropathies caused by oxaliplatin were associated with deterioration of QoL. Patients with peripheral neuropathy had lower QoL on the symptoms scale, especially severe nausea, vomiting, and diarrhea. There was also a decrease in emotional and cognitive functions. Storey et al. [28] reported that peripheral neuropathy occurred in 28% of patients after chemotherapy, accompanied by a loss of function in 7% at 12 months. Kim et al. [29] reported that 59.41 points of health-related QoL, 73.29 points of functional scale, and 26.72 points of symptom scale were scored. After six cycles of the adjuvant chemotherapy, health-related QoL scored 57.74 points, functional scale 84.90 points, and symptom scale 16.49 points in this study. Our patients scored 10 points higher over the QoL on the functional and symptoms scales, and this is likely because many patients with palliative chemotherapy had been included in previous studies. Peripheral neuropathy has no obvious preventive and therapeutic methods, so decreased QoL may continue in these patients. Therefore, efforts to improve QoL through appropriate supportive care, avoidance therapy, and encouragement of patients are needed.
Thoresen et al. [9] reported that if colon cancer patients gain 5% of their weight after 3 months, general health status, functional scale, insomnia, and appetite loss improve. On the contrary, if patients lost more than 5% of their weight, physical, role, and social functioning scaled low and fatigue was worse. In this study, after 6 cycles of adjuvant chemotherapy, if patients gained more than 5% of their weight, symptom scales scored low. In particular, patients complained less symptoms of fatigue (P=0.03) and pain (P=0.047) on the symptom scale. Furthermore, those patients with delayed chemotherapy were small in number (P=0.012). All patients had a follow-up period of 3 months, similar to that in Thoresen’s study. The main cause of weight gain under 5% in the univariate analysis was delayed chemotherapy more than 2 times, and adverse events in chemotherapy may cause decreased QoL.
This study collected data prospectively but the number of patients is small. Limitations of this study included peripheral neuropathy, which occurred on average after 8 cycles of chemotherapy and that QoL during the early stages of adjuvant chemotherapy was the only point analyzed during treatment. Therefore, additional long-term follow-up of many subjects after 12 cycles adjuvant chemotherapy with curative resection are needed for accurate evaluation for QoL.
There is no difference in QoL between the time before and after chemotherapy. Initial adverse effects of oxaliplatin were gastrointestinal related symptoms such as nausea. After 6 cycles of chemotherapy, QoL related factors were age over 70, weight gain of more than 5%, chemotherapy delayed more than twice, and peripheral neuropathy. In order to improve QoL during chemotherapy medications for the relief of adverse effects caused by chemotherapy, self-management education, active support and intervention by physicians are necessary.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the research grant of the Chungbuk National University in 2014.