위암환자에서 수술 전 neutrophil-to-lymphocyte ratio가 예후인자로 의미 있는 그룹은?
In which group of gastric cancer patients is the preoperative neutrophil-to-lymphocyte ratio a significant prognostic factor?
Article information
Trans Abstract
Purpose
Several recent studies have reported on the clinical importance and prognostic significance of neutrophil-to-lymphocyte ratio (NLR) in gastric cancer. The objective of this study was to identify the subgroups of patients with gastric cancer for which the preoperative NLR was prognostically significant.
Methods
Data from 870 patients who were among those who had undergone surgery for gastric cancer between August 2005 and December 2013 were evaluated. Receiver operating characteristic curve analysis was used to determine the cut-off value for NLR. The patients were classified into high-NLR (NLR≥1.7) and low-NLR (NLR<1.7) groups, and survival analysis of subgroups of gastric cancer patients was performed.
Results
Univariate analysis identified age, gender, tumor location, tumor histology, tumor, node, metastasis (TNM) stage, and NLR as significant prognostic factors. Multivariate analysis identified age, TNM stage, and NLR as significant prognostic factors. In subgroup analysis, NLR was a significant prognostic factor except group of TNM stage I, II with age younger than 70 years.
Conclusion
Except group of TNM stage I, II with age younger than 70 years, careful postoperative follow-up is warranted for those patients with elevated NLR.
INTRODUCTION
Gastric cancer is the second most common malignancy and the third leading cause of cancer death in Korea [1]. Several studies have attempted to determine the prognostic value of various markers of systemic inflammatory responses for certain groups of cancer patients. These markers include intercellular adhesion molecule-1 and the neutrophil-to-lymphocyte ratio (NLR), both of which are affected by cytokines [2]. The NLR can be easily calculated from the differential white blood cell count, and can be used to evaluate cell-mediated immune responses [3]. In addition, the preoperative NLR is an indicator of the inflammatory state, clinical stage, and survival rate of patients with liver, lung, colon cancer [4-6].
Recently, several studies have reported on the clinical importance and prognostic significance of NLR in gastric cancer. They found that an elevated preoperative NLR predicted poor overall survival [7-9]. From our institution, we reported that a preoperative NLR greater than 1.7 was significant prognostic factor [10]. However, we did not evaluate the significance of NLR for subgroups of gastric cancer patients. We supposed that NLR would not be a significant prognostic factor in all subgroups of gastric cancer patients. The aim of this study was to identify the subgroups of patients with gastric cancer for which the preoperative NLR was prognostically significant.
METHODS
Patients
Data from 870 patients who were among those who had undergone elective surgery for gastric cancer at Konkuk University Medical Center, Seoul, South Korea, between August 2005 and December 2013 were evaluated. The study patients met the following inclusion criteria: primary gastric cancer, no preoperative chemotherapy, no distant metastasis, R0 resection (no residual macroscopic or microscopic tumor), and more than 15 examined lymph nodes. The patients’ clinical information and follow-up data were obtained from their electronic records. The clinicopathological data included the following: patient demographics (age and gender), preoperative NLR, pathological characteristics of the tumor (size, location, histology, tumor, node, metastasis (TNM) stage according to the Seventh Edition of the American Joint Committee on Cancer TNM classification system, and the number of examined lymph nodes), and follow-up data (follow-up duration and survival status).
Methods
Peripheral blood samples were obtained from each patient during the 2 weeks before surgery. The absolute number of white blood cells and the differential cell count were determined manually using a hemocytometer, and the NLR was defined as the absolute neutrophil count divided by the absolute lymphocyte count.
As previous study, receiver operating characteristic (ROC) curve analysis was used to determine an appropriate cut-off value for the NLR. The optimal value was 1.7, and the study patients were classified into a high-NLR (NLR≥1.7) group and low-NLR (NLR<1.7) group [10].
Patients were evaluated according to tumor location, which was determined from the surgical records, as follows: upper third, middle third, and lower third. Histological subtypes were categorized as follows: differentiated (papillary adenocarcinoma, well differentiated tubular adenocarcinoma, and moderately differentiated tubular adenocarcinoma) and undifferentiated (poorly differentiated tubular adenocarcinoma, signet ring cell carcinoma, and mucinous adenocarcinoma). The duration of patient follow-up was determined from the date of surgery to the last follow-up date or date of death.
Statistical analysis
The independent t-test, chi-square test, and Fisher exact test were performed to compare the clinicopathological characteristics of the high-NLR patients and low-NLR patients. Cumulative survival rates were compared using the Kaplan-Meier method and the log-rank test. The Cox proportional hazards model was used for multivariate analysis to identify independent prognostic factors. Data were analyzed using IBM SPSS ver. 20 (IBM, Armonk, NY, USA), and P-values less than 0.05 were considered to be statistically significant.
RESULTS
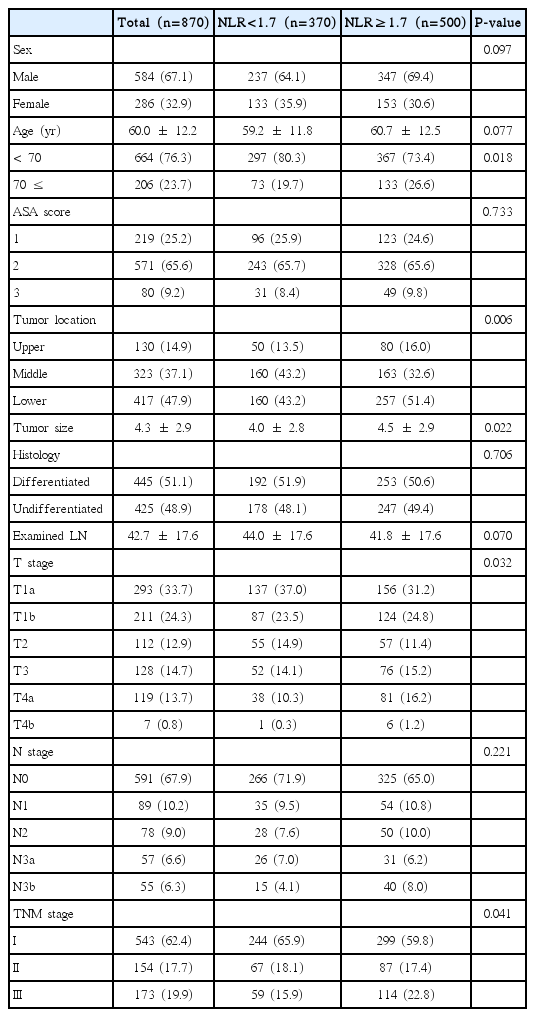
The median follow-up period of all 870 patients was 39.0 months (range, 3.3 to 104.4 months). The demographics and clinicopathological characteristics of the patients according to NLR level are summarized in Table 1. The differences in age (P=0.018), tumor location (P=0.006), tumor size (P=0.022), T stage (P=0.032), and TMN stage (P=0.041) between the patients with NLR <1.7 or ≥1.7 were statistically significant. There were no differences in gender, American Society of Anesthesiologists (ASA) scores, or pathological features (histology, number of examined LNs, and N stage).
Fig. 1 shows the Kaplan Meier curve of the overall survival of patients stratified by age, gender, tumor location, histology, TNM stage, and NLR as significant prognostic factors. The differences between patients stratified by these variables were significant. However, multivariate analysis identified TNM stage, NLR, and age as significant independent prognostic factors (Table 2).
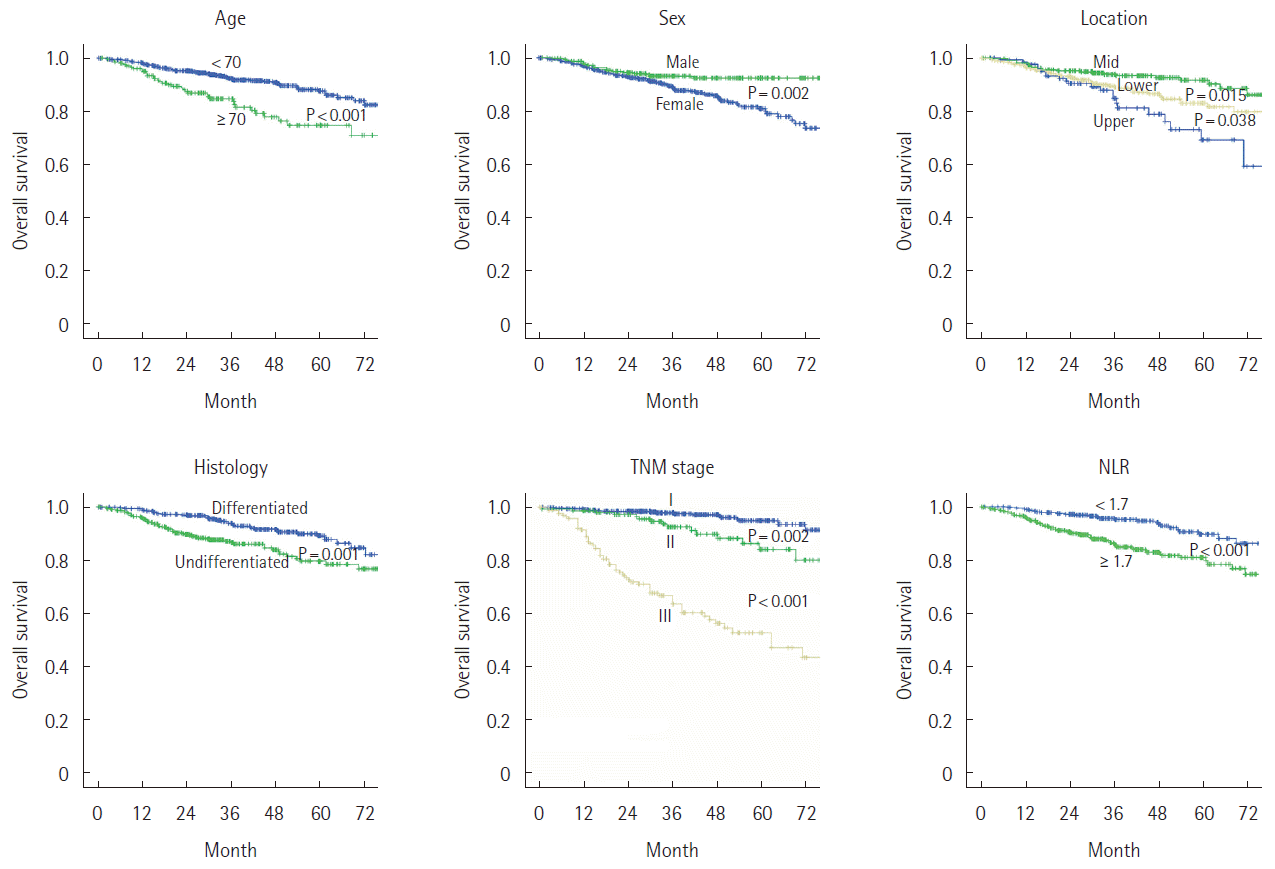
Kaplan Meier survival curve for significant variables in univariate analysis. TNM, tumor node metastasis; NLR, neutrophil-to-lymphocyte ratio.
When patients groups were stratified by age, gender, histology, and tumor location, NLR was a significant prognostic factor in each group. However NLR was not statistically significant in groups with TNM stage I, II (P=0.178, 0.139) (Fig. 2). In patient group of TNM stage I, II with age <70 years, NLR was not statistically significant (P=0.478), and in group of TNM stage I, II with age ≥70 years, NLR was significant prognostic factor (P=0.035) (Fig. 3). Other subgroup analysis demonstrated that NLR was a significant prognostic factor.
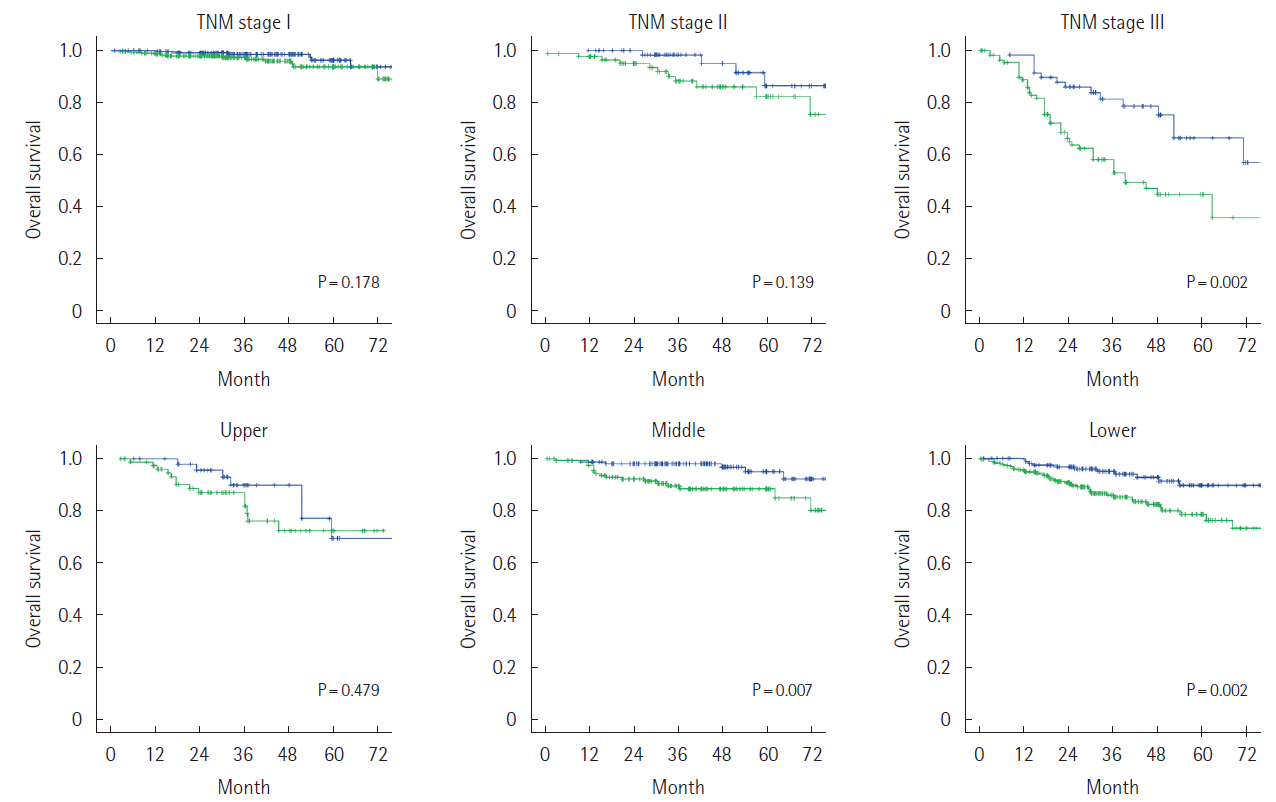
Kaplan Meier curve for subgroup analysis (blue line, low NLR group; green line, high NLR group). TNM, tumor node metastasis; NLR, neutrophil-to-lymphocyte ratio.
DISCUSSION
Inflammation has a fundamental role in the pathogenesis and progression of many disease states, including cancer [11]. An association between inflammation and cancer was first reported by Rudolf Virchow in 1863, who suggested that a lymphoreticular infiltrate reflected the chronic inflammation at the site of cancer [12]. Recent evidence has shown that a systemic inflammatory response is related on the prognostic value in various cancers [13].
The NLR is a measure of the relative difference of between neutrophil and lymphocyte counts. Neutrophilia, by providing an adequate tumor microenvironment via the production of cytokines and chemokines, may lead to the development and progression of cancer [14]. Likewise, the tumor microenviroment may have an effect on invading leukocytes by reinforcing angiogenesis [15]. Unlike neutrophils, lymphocytes are important components of cancer immunosurveillance, and suppress tumor progression [16]. A decreased lymphocyte count in patients with advanced gastric cancer predicts poor survival and indicates depressed innate cellular immunity [17]. Therefore, an elevated NLR is a marker of systemic inflammation, which reflects an imbalance between a protumor inflammatory state (neutrophilia) and antitumor immune state (lymphopenia). Moreover, inflammation not only has a fundamental role in the development of tumors but also is extremely important in the progression of various malignant diseases such as gastrointestinal tract cancer [18].
Since the NLR is an indicator of both the inflammatory and immune state of a patient with cancer, it has been considered to be an easily available and reliable predictor of the survival of patients with liver, lung, colorectal, breast, and renal malignancies [4-6,19,20]. Several studies have shown that an elevated preoperative NLR has been a predictor of poor survival in these cancers.
A previous study at our institution that evaluated the relationship between the preoperative NLR and overall survival in gastric cancer found that a preoperative NLR ≥1.7 was a significant prognostic factor [10]. In 2009, the proportion of gastric cancer patients in Korea with early gastric cancer was 66.7% [21]. Whether or not an elevated NLR is a significant marker for the poor survival of patients with early gastric cancer was unclear. Therefore, we conducted a study to investigate if there were patient subgroups for which the preoperative NLR was a significant prognostic factor, and found NLR was a significant prognostic factor except group of TNM stage I, II with age younger than 70 years. Maybe it can be explained by the low aggression of initial stage of tumor and relatively high immunocompetence in younger age. However, it is just hypothesis and it should be elucidated through further study.
Our study has some limitations. First, our study was retrospective, with a relative small number of patients at a single institution. Second, we did not take into account inflammatory conditions that might have affected the NLR of study patients. However, because elective surgery was usually performed in stable patients without inflammatory condition, we thought that the effect to NLR was minimal.
In conclusion, our study verified previous studies showing that an elevated preoperative NLR in patients with gastric cancer is associated with worse survival. Except group of TNM stage I, II with age younger than 70 years, careful postoperative follow-up is warranted for those patients with elevated NLR. NLR measurements may have a role in the delivery of personalized cancer care.
Notes
No potential conflict of interest relevant to this article was reported.