Impact of proximal resection margins on oncologic outcomes for patients with sigmoid colon cancer
Article information
Abstract
Purpose:
The purpose of this study was to evaluate the association between proximal resection margin (PRM) length and oncologic outcomes in sigmoid colon cancer.
Methods:
This was a retrospective cohort study on patients with sigmoid colon cancer between 1996 and 2009 and treated by curative primary resection. Tumor location was classified into two groups: SDPS, which was located in the proximal sigmoid colon or the sigmoid-descending junction; and MDS, which was located in the mid and distal sigmoid colon.
Results:
The mean PRM length was 8 cm (range, 6 to 11.5 cm). A total of 132 patients (11.7%) had a PRM<5 cm, which was more common in the SDPS group. Patients with PRMs<5 cm and ≥5 cm did not differ in age, sex, size of tumor, histologic differentiation, stage, and number of harvested lymph nodes. Five-year relapse-free survival (RFS) was not affected by PRM length among patients and was not associated with RFS according to the Cox regression analysis.
Conclusion:
PRM length was associated with tumor location within the sigmoid colon but not with the oncologic outcome.
INTRODUCTION
Although significant advances have been made in colorectal cancer management, major changes have been suggested for rectal cancer. The principals of colon cancer surgical resection have remained fairly constant. The key to achieving oncologically safe resection is to obtain clear resection margins and removal of the locoregional lymph node bearing mesentery.
Apart from a few challenging cases, colon cancer is relatively easy to handle and it has a wide operative field compared to rectal cancer. Therefore, concerns regarding bowel margins are limited to only a few unusual cases. The adequacy of proximal and distal resection margins for colon cancer is primarily defined by vascular ligation and hence the adequacy of the vascular supply to the intended anastomotic segment. Although not clearly defined, it is generally agreed that 5 cm bowel margins are sufficient to allow resection of mural tumor spread [1]. Some data suggests that mural tumor migration is rarely greater than 2 cm, either proximally or distally, to the palpable tumor edge [2]. In addition, there was a report postulating that adequate clearance of the mesocolon containing the vascular and lymphatic system is more important for colon cancer surgery [3].
The proximal bowel resection margin is one of the surrogates of surgical quality: however, in some cases, it is difficult to obtain ≥5 cm proximal resection margins (PRM). For example, in patients with proximal sigmoid colon cancer, ≥5 cm PRMs cannot be obtained by anterior resection without splenic flexure mobilization. Therefore, we need to assess the influence of PRMs on oncologic outcomes to establish a surgical standard for colon cancer.
The aim of this study was to assess the association of the length of the PRM with other surrogates of surgical qualities such as the number of retrieved lymph nodes, stage, distal resection margin and to analyze the influence of PRM on oncologic outcome.
METHODS
Patients
The data of 1,131 patients with sigmoid colon cancer was analyzed. All patients underwent curative resection between January 1996 and December 2009 at the Asan Medical Center. Patients with concurrent distant metastasis or disease confined within the mucosa at diagnosis, those with concurrent malignancy, urgent surgery, who underwent subtotal or total colectomy, and those with metachronous or synchronous colorectal cancer were excluded from this study.
The study was approved by the Asan Medical Center Institutional Review Board. Patients were classified into two groups according to tumor location within the sigmoid colon: the SDPS group consisted of patients with the tumor located at the proximal sigmoid colon or sigmoid-descending junction. The MDS group consisted of patients with mid and distal sigmoid colon cancer. The proximal sigmoid colon was defined as the sigmoid colon proximal to the 2nd branch of the sigmoid artery. The sigmoid-descending junction was defined as the distal area leaving the colic artery origin. The distal sigmoid colon was defined as the sigmoid colon distal to the 2nd branch of the sigmoid artery.
Histopathology
Proximal and distal bowel margins were measured macroscopically in pinned formalin-fixed specimens. The PRM was defined as the closest distance between the proximal border of the gross tumor and the edge of the proximal resection. Anastomoses were performed using a stapling device in most sigmoid colon cancer cases, and the cutting edges of the doughnut were not included in the measurement of the proximal and distal resection margins.
Patients were classified into two groups according to PRM length: <5 cm PRM and ≥5 cm PRM. The oncologic endpoint was relapse-free survival (RFS), which was defined as the time between surgery and tumor recurrence.
Statistical analysis
Patient and tumor related variables were characterized using descriptive statistics and comparisons were performed using the chi square test for proportions and the t-test for mean values. Five-year disease-free survival rates were determined by the Kaplan-Meier method and compared according to the length of the PRM with the log-rank test. Cox proportional hazards regression analysis was performed to calculate hazard ratios and 95% confidence intervals for demographic and clinicopathological variables. A P<0.05 were considered statistically significant. All analyses were performed using SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Clinicopathologic characteristics of the patients
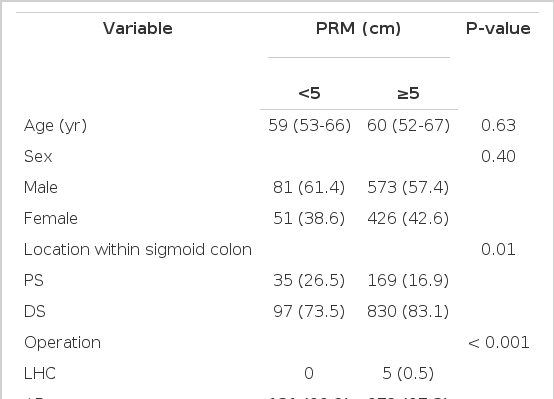
The median age was 59 years (interquartile range [IQR], 52 to 67 years). Males were more common than females (654, 57.8%). Most tumors were located in the mid to distal sigmoid colon (82.8%). Anterior resection was the most commonly performed procedure (1,102, 97.4%). Left hemicolectomy was performed in six patients with the disease located in the proximal sigmoid colon. The median number of retrieved lymph nodes was 15 (IQR, 10 to 21). The median PRM length was 8 cm (IQR, 6 to 11.5). In 132 patients (11.7%), the PRM was <5 cm. Clinical and tumor characteristics according to PRM are shown in Table 1. Proximal sigmoid colon involvement was more common among patients with <5 cm PRM. Pathologic variables were not different between the two groups. No relationship was observed between PRM length and the number of retrieved lymph nodes, length of the distal resection margin, and size of the tumor (Fig. 1).
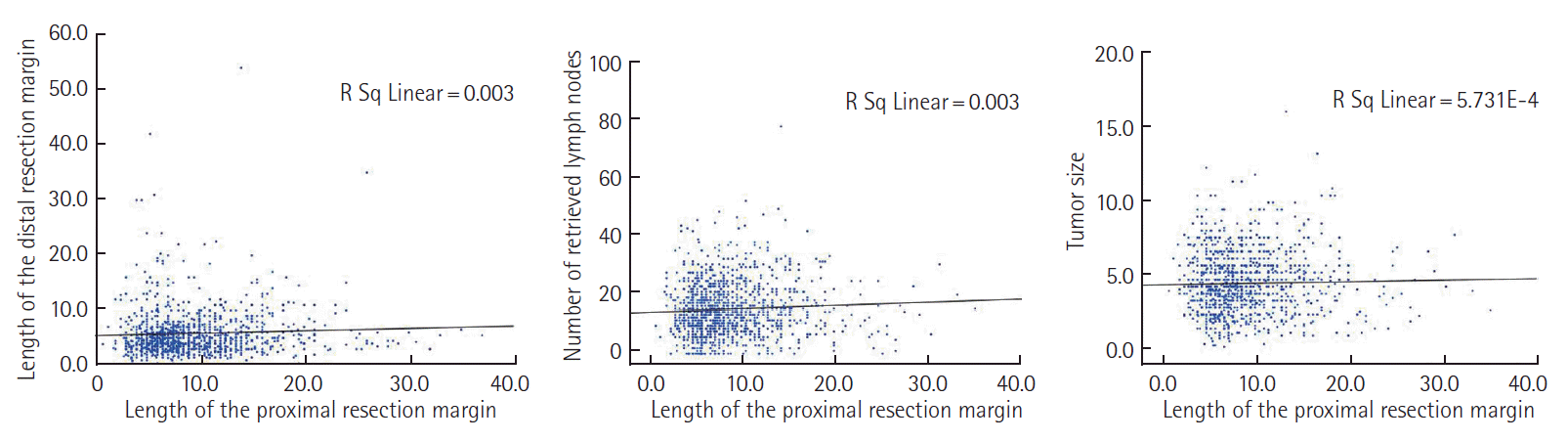
Correlation between proximal resection margin length. (A) The length of the distal resection margin, (B) the number of retrieved lymph nodes, (C) the size of the tumor. The length of the proximal resection margin was not associated with the length of the distal resection margin, number of retrieved lymph nodes, and tumor size.
Surgical and oncologic outcomes
Median follow-up was 60 months (IQR, 43 to 79 months). Anastomotic leakage developed in one patient with a PRM length ≥5 cm. Overall recurrence occurred in 168 patients (14.9%). Distant metastases including hematogenous, distant lymph nodes, and peritoneal seeding occurred in 162 patients (14.3%).
Local recurrence occurred in nine patients (0.8%). Among them, four patients had anastomotic recurrence and five had regional lymph node recurrence. Among patients with anastomotic recurrence, only one patient had PRMs <5 cm. Recurrence rates were significantly different according to the pathologic stage: 5.4% for stage I, 10.6% for stage II, and 26.2% for stage III patients. Local recurrence rates were not affected by the pathologic stage. Fiveyear RFS for all patients was 84.9%. Five-year RFS according to the pathologic stage was as follows: 94.5 % for stage I, 89.0% for stage II, and 73.8% for stage III (P<0.001).
Impact of PRM length on oncologic outcome
The 5-year RFS was not different according to the PRM length. By stage, 5-year RFS was not associated with the PRM length (Fig. 2). When analyzing the relation between PRM length and RFS in the SDPS group, we noted that 5-year RFS was not affected by PRM length (P=0.79) (Fig. 3). The prognostic significance of PRM length for RFS was evaluated. On multivariate analysis, pathologic stage and lymphovascular invasion were independent prognostic factors for RFS among all patients. PRM length was not associated with RFS by multivariate analysis (Table 2).
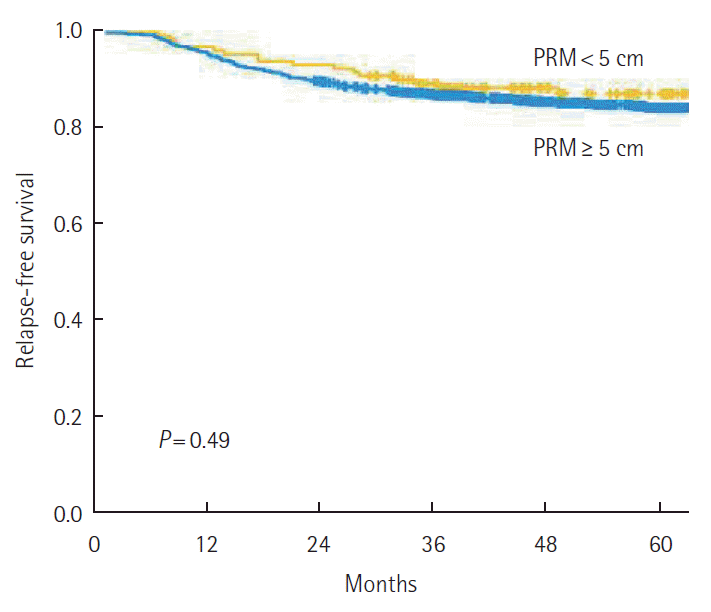
The 5-year relapse-free survival (RFS) according to proximal resection margin (PRM) length (<5 cm vs. ≥5 cm). The 5-year RFS was not affected by PRM length.
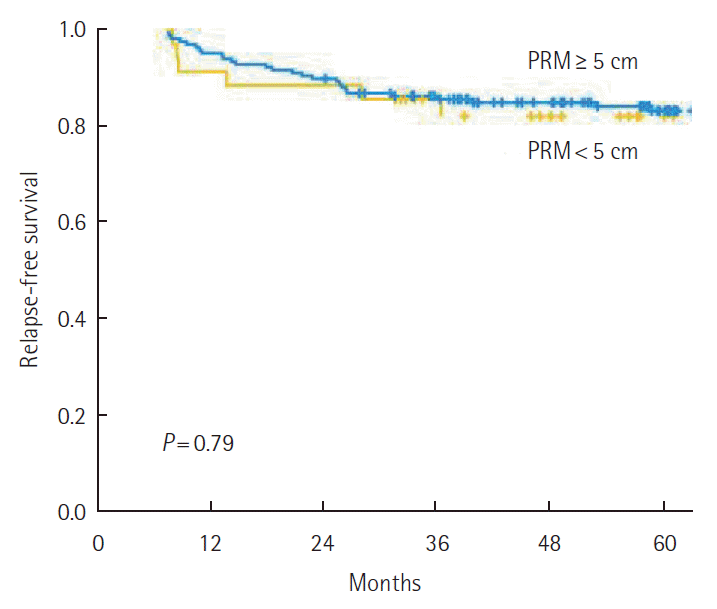
The 5-year relapse-free survival among patients with a tumor located in the proximal sigmoid colon or the sigmoid-descending junction according to proximal resection margin (PRM) length.
DISCUSSION
In the present study, we determined that PRM length is not associated with oncologic outcome among patients with sigmoid colon cancer. For sigmoid colon cancer patients, the PRM is determined according to the level of ligation of the inferior mesenteric artery because this affects the adequacy of the vascular supply to the intended anastomotic segment. The relation between PRM length and oncologic outcome has not been taken into consideration in these patients. The intramural spread of colorectal cancer up to 4 cm is well described in previous pathological studies and forms the basis of the “5 cm rule” in determining resection margins for rectosigmoid and rectal cancer [1,4]. However, the length of the distal resection margin has been challenged. A previous study showed that 94% of patients with rectal cancer had distal intramural spread less than 2.5 cm, suggesting that patients with intramural spread greater than 2.5 cm were those with poorly differentiated tumors [5].
It is now accepted that extending the resection margins further to include intramural spread may not affect survival rates, since most of these patients are more likely to die from the presence of distant metastases than from local recurrences. Hence the presence of distant intramural spread may be an indicator of poor prognosis. Since then, many studies have been performed to prove that shorter distal resection margins are acceptable in terms of oncologic outcomes [6-8] in an effort to preserve the sphincter, especially in patients with rectal cancer.
PRM, however, has not been in the consideration because enough PRM could be easily got for patients with colon cancer. In some case, unnecessarily immoderate PRM might be obtained for patients with sigmoid or rectal cancer without evidence of benefit of excessive PRM.
Studies have been performed to determine the most adequate surgery for patients with colon cancer. Resection of the intact mesocolon has been suggested based on the concept that the colonic mesentery or mesocolon contain the vascular and lymphatic drainage systems of the colon and therefore adequate clearance is likely to have the same oncologic benefit as total mesorectal excision [3]. The authors reported that meticulous mesocolic plane surgery is associated with improved overall survival [9]. They did not indicate bowel resection margins as one of the determinants of oncologic outcome. A second analysis of the COST trial [10] insisted that in the setting of technical credentialing and monitoring, surgical quality surrogates, including proximal and distal resection margins, mesenteric length, and number of retrieved lymph node, did not correlate with cancer outcomes. The “rule” of PRM length needs to be reconsidered, based on these results.
Resection of enough long proximal resection margin is not difficult for patients with colon cancer most of the cases. However, we would encounter situation whether to resect excessive bowel or get less enough proximal resection margin in some cases such as patients with proximal sigmoid colon or sigmoid-descending junction colon cancer. Proximal resection margin was determined by the adequate vascular supply to anastomosis. Anastomotic leakage occurred in one patient with ≥5 cm PRM in the present study. Short PRM did not associate with anastomotic complication.
Luminal recurrence was one of the concerns for short PRMs as the length of the PRM was guided by the extent of the intramural spread of the tumor [1]. In the present study, most recurrences (96.4%) occurred among patients with distant metastases. Anastomotic recurrence developed only in one patient who had ≥5 cm PRM. Therefore, a shorter PRM does not appear to increase the risk of local recurrence. PRM length was not associated with local recurrence or anastomotic complications. Rather it would be better to be evaluated in terms of association with systemic metastasis. PRM length was associated with the extent of mesocolic excision, including lymphatic and vascular drainage. We analyzed the association between PRM length and the number of retrieved lymph nodes and determined that these two factors were independent. Tumor size, lymphovascular invasion, perineural invasion, distal resection margin length, and pathologic stage were also independent of PRM length, meaning that a shorter PRM was not associated with less extensive resection in terms of the pathologic surrogate of surgical extent.
We analyzed the influence of PRM length on surgical and oncologic outcomes according to tumor sub-location because short PRMs could be more common among patients with proximal sigmoid or sigmoid-descending junction cancer. As expected, patients with a PRM length <5 cm were more frequently identified in the SDPS group, although surgical and oncologic outcomes did not vary according to PRM length in relation to tumor sub-location.
This study has a few limitations that should be considered when interpreting the results. There remains a potential for both referral and selection bias, as the study was a retrospective and observational cohort study performed at a single-institution. Despite the large sample size, the relatively small number of patients in the PRM <5 cm sub-group did not make a significant impact on the RFS comparisons. In addition, we examined PRM length as a categorical variable with a cut-off of 5 cm, as this was the recommended guideline, but there is a possibility that this does not reflect the continuous impact of PRM on oncologic outcomes. In addition, we could not suggest the adequate proximal margin which did not compromise oncologic outcome because of small number of subgroup with shorter than cm PRM. However, we did evaluate the relationship between PRMs and other pathologic variables in order to compare surgical extents according to PRM length. From these observations we can conclude that a shorter PRM was not associated with anastomotic complications, increased local recurrence, or poor oncologic outcomes.
In conclusion, this large single-institution study on sigmoid colon cancer patients demonstrated that a PRM length <5 cm is not considered a less extensive resection. PRM length did not affect the oncologic outcomes. This relationship was also observed among patients with proximal sigmoid/sigmoid-descending junction cancer. In addition, regardless of PRM length, most recurrences were systemic metastasis and were most tightly associated with the pathologic stage. In conclusion, excessive bowel resection in order to obtain longer PRMs should be done in moderation on these patients.
Notes
No potential conflict of interest relevant to this article was reported.