INTRODUCTION
Intraductal papillary mucinous neoplasms (IPMNs) are cystic neoplasms that grow within the ductal system of the pancreatic gland [1]. They exhibit varying malignant potential, requiring different treatments ranging from surveillance to surgical approaches [2]. Invasive IPMNs are at imminent risk of malignant degeneration, necessitating surgical resection for treatment. Also, the overall survival rate after resection of invasive IPMNs is getting greater than that of pancreatic ductal adenocarcinoma [3]. Several guidelines recommend surgical resections based on clear indications, including enhancing mural nodule >5 mm, dilatation of the main pancreatic duct >10 mm, and obstructive jaundice in the context of a cystic mass in the head of the pancreas [1].
While there remains some debate surrounding the optimal management of invasive IPMNs, the role of adjuvant or neoadjuvant therapy for IPMN has still been conflicting [4]. However, the current literature lacks substantial discussion regarding the use of neoadjuvant therapy in cases where surgical resection may be challenging or contraindicated. The 2018 European Guidelines made no recommendation for neoadjuvant therapy only for locally advanced IPMN (grade 2c, strong agreement) [4]. The lack of evidence might underscore the need to explore alternative treatment strategies that may be applicable in such circumstances.
In light of this, we present the first intriguing case of using neoadjuvant therapy in the management of a patient with an invasive IPMN that complete margin-negative surgical resection was deemed difficult at the initial diagnostic stage. It would highlight the potential benefits and considerations of neoadjuvant chemotherapy in advanced invasive IPMN. Our case report aims to contribute to the growing body of evidence surrounding the management of IPMNs and to stimulate further discussion on the role of neoadjuvant therapy in this context. This report was approved by the Institutional Review Board of Severance Hospital (2023-1444-001). The informed consent was waived.
CASE REPORT
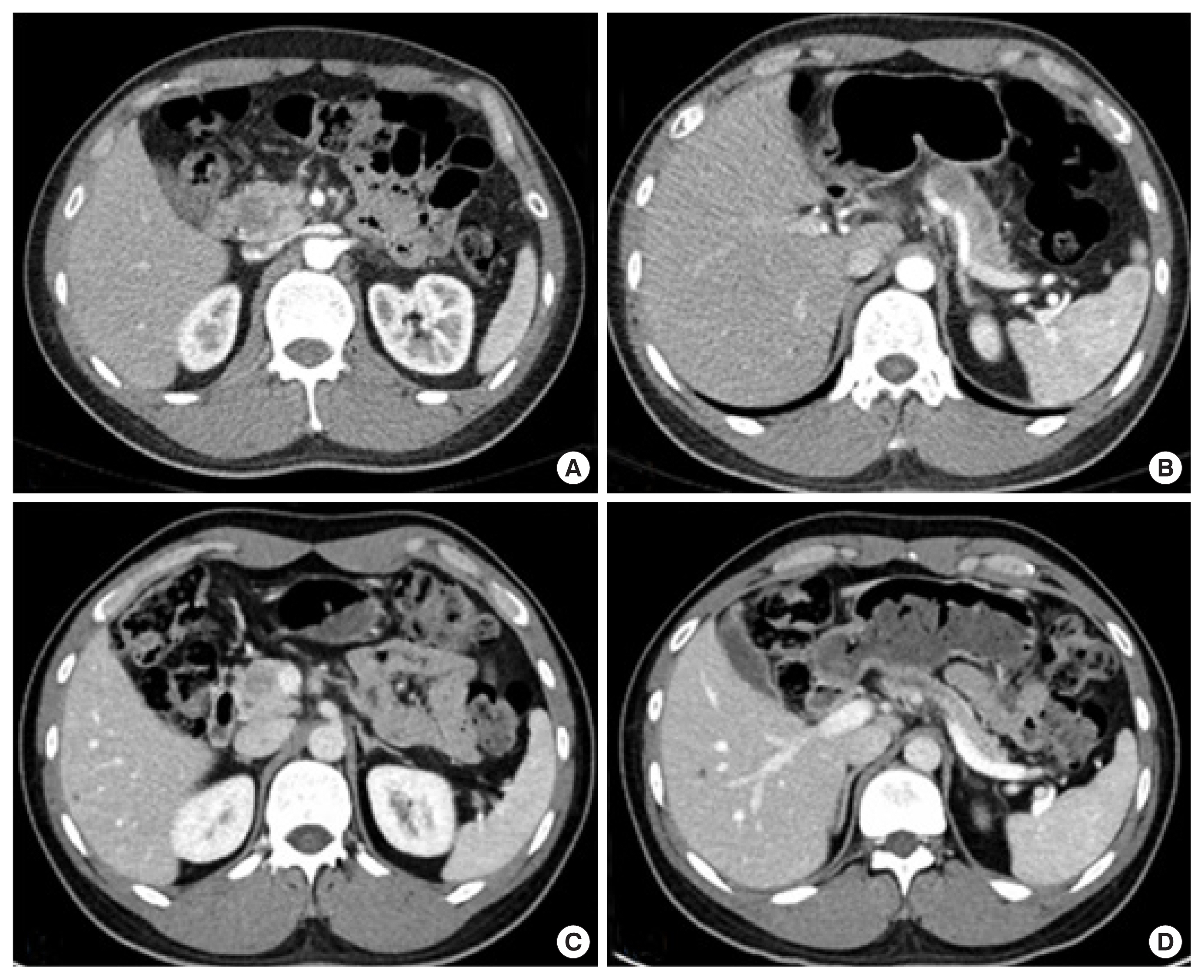
A 34-year-old male patient was admitted to our hospital for the examination of a suspicious pancreatic luminal mass in the pancreas from head to body. It had been monitored at the primary hospital since 3 years ago, following the first episode of recurrent pancreatitis. He had no other surgical or medication history but a family history of pancreatic cancer from his father; serum carbohydrate antigen 19-9 (CA19-9), 8.2 U/mL carcinoembryonic antigen (CEA), 0.41 ng/mL. Outside images of computed tomography (CT) and magnetic resonance imaging (MRI) identified a mass filling in the pancreatic duct from the head to the body portion and dilatation of the upstream pancreatic duct (Fig. 1A and B). Endoscopic ultrasound (EUS) showed a pancreatic luminal mass from head to body, and EUS-guided fine needle aspiration cytology revealed the atypical proliferation of columnar cells with low-grade dysplasia. According to these studies, the patient was diagnosed with diffuse main duct IPMN. Positron emission tomography-CT, CT, and MRI identified no evidence of distant metastasis. However, MRI showed a 2 cm-sized deformity in the main portal vein (PV) and portomesenteric junction.
Considering current surgical techniques, the tumor was thought to be resectable [4], but nearly the total pancreas should be removed for curative margin-negative resection. In addition, R1 resection was expected due to the marked tumor distribution along the main pancreatic duct. Therefore, the patient underwent neoadjuvant chemotherapy with the FOLFIRINOX drugs (2,800 mg/m2 of 5-fluorouracil, 400 mg/m2 of leucovorin, 180 mg/m2 of irinotecan, and 85 mg/m2 of oxaliplatin) once every 2 weeks. After five cycles of FOLFIRINOX, follow-up CT showed a decreased size of the intraductal mass in the pancreatic duct without any evidence of the major vessel invasion and the improvement of the distal pancreatic duct dilatation as noted (Fig. 1C and D).
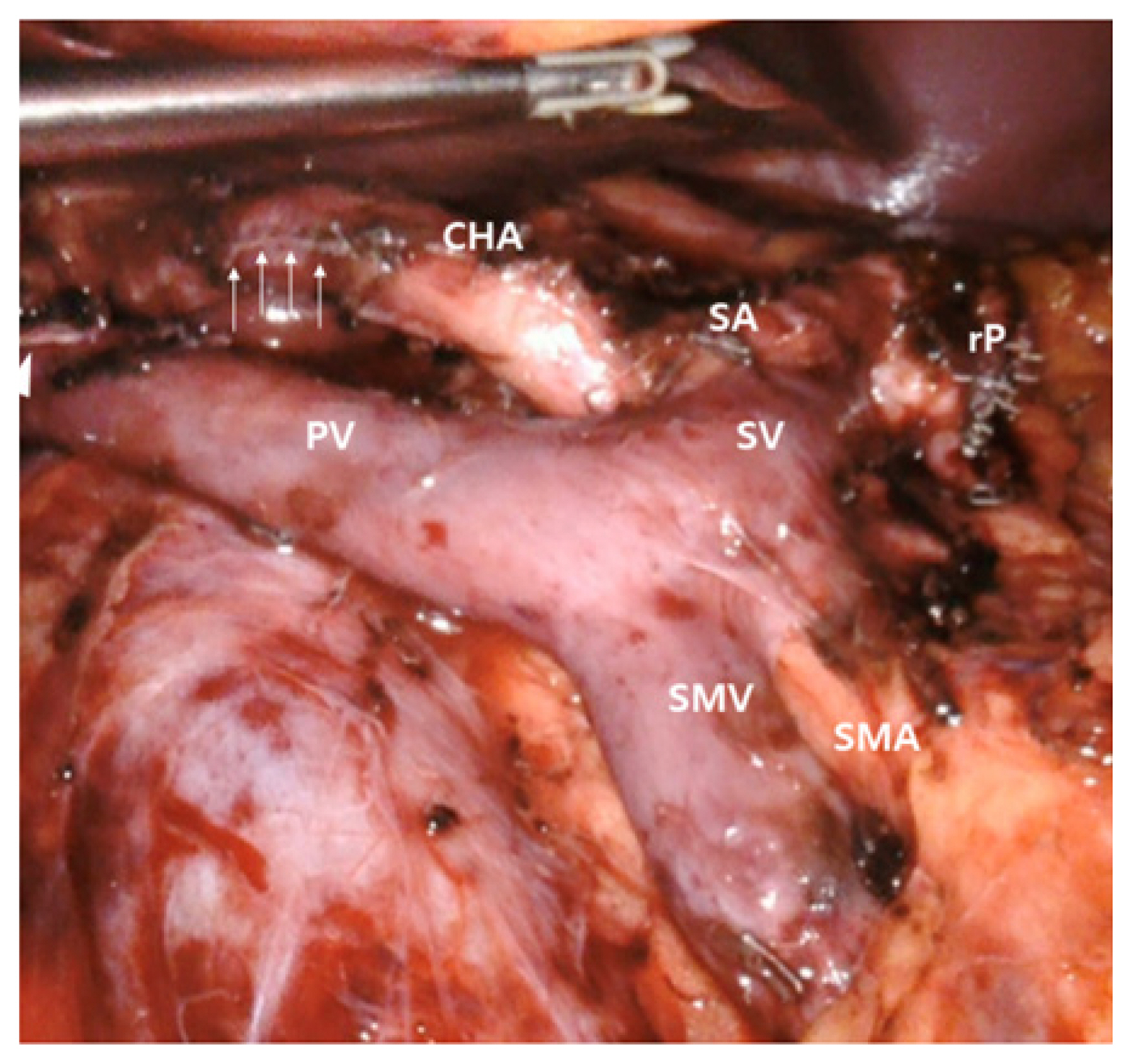
The patient underwent a robotic pylorus-preserving total pancreatoduodenectomy on November 7, 2022. The pancreas was divided more than 3 cm from the splenic artery origin, based on the CT scan (Fig. 2). There were severe adhesions around the lesions, but no invasion. The soft tissue dissection was available around the lateral border of the superior mesenteric artery (SMA), as well as around the celiac artery and the common hepatic artery. According to the initial CT scan, pancreatic resection was done at the body portion about 3 cm apart from the origin of the splenic artery for enough tumor-free margin. The intraoperative frozen section concluded that all resection margins were negative. Subsequent pancreaticojejunostomy, hepaticojejunostomy were done by robotic approach after laparoscopic resection of the tumor [5]. Duodenojejunostomy was performed through a small extended periumblical wound by manual approach.
The pathological diagnosis of the tumor was intraductal tubulopapillary neoplasm with associated invasive carcinoma (ICD-O code: 8503/3) [6]. The lesion was confined within the pancreas and peripancreatic soft tissue, which measured 2.6×1.8×1.5 cm. Lymphovascular and perineural invasions were not identified, while adjacent lymph nodes were all free from tumors. Resection margins were all free from tumors, especially including 1.5 cm-sized safety margin of the SMA and the 0.2 cm-sized safety margin of the superior mesenteric vein/PV groove. A total of seven lymph nodes were identified without any positive nodes (ypN0). Mesenteric soft tissue was also negative for carcinoma cells. The pathological stage of the lesion was ypT2N0 (stage I), according to the post-neoadjuvant treatment staging (ypTNM) system of the American Joint Committee on Cancer 8th edition [7].
The patient’s postoperative recovery was uneventful, and he was discharged from the hospital without any complications 14 days after surgery. Over the 5 months following surgery, the patient underwent seven cycles of FOLFIRINOX (2,800 mg/m2 of 5-fluorouracil, 400 mg/m2 of leucovorin, 180 mg/m2 of irinotecan, and 85 mg/m2 of oxaliplatin) as adjuvant chemotherapy. On April 17, 2023, after the latest therapy, his glucose level was 100 mg/dL. Also, both tumor markers, CEA and CA19-9, were 3.72 ng/mL and 5.3 U/mL, respectively. Those levels were all stable, which indicates no recurrence of the tumor or specific complications.
DISCUSSION
There are varying treatment options for the IPMN, depending on its grade [4]. Surgery is the most important strategy to treat high-grade IPMN, with a wide resection margin [4,6]. However, in some patients, recurrence can develop even after surgery, with progression to unresectable invasive IPMC from local spread or metastases [3]. In order to avoid those outcomes, extra chemotherapy may be needed before or after the surgery [4,6]. In this point of view, we present a case report of neoadjuvant therapy with the FOLFIRINOX regimen in a patient with invasive IPMN. Both surgical resection and neoadjuvant chemotherapy were employed in this case, but our discussion will primarily focus on the necessity of neoadjuvant chemotherapy.
In this case, there were several reasons why neoadjuvant chemotherapy was conducted before elective surgery. First, total resection of the pancreas was needed to acquire a safe resection margin. Also, according to initial imaging studies, the lesion was abutting the PV, resulting in insecure resectability. Thus, it was anticipated that reducing the size and deformity would be necessary for a better surgical outcome. After five cycles of FOLFIRINOX, the size of the intraductal mass and the pancreatic duct dilatation were critically decreased. Also, the abutment and the deformity within the main vessels were significantly improved.
The role of neoadjuvant therapy in the management of invasive IPMN is not well defined. Existing studies on neoadjuvant chemotherapy in the management of pancreatic tumors have mainly focused on pancreatic cancer. According to van Dam et al. [8], neoadjuvant chemotherapy can improve survival in patients with resectable and borderline resectable pancreatic cancer. Also, according to Oba et al. [9], regarding the efficacy of different neoadjuvant chemotherapy regimens in pancreatic cancer, patients receiving FOLFIRINOX had longer overall survival compared to those treated with gemcitabine. Based on the results of these studies, we used the FOLFIRINOX regimen.
To the best of our knowledge, this is the first report of neoadjuvant chemotherapy reports for invasive IPMN. Although further research is needed to establish standardized guidelines for its application, our findings suggest that neoadjuvant therapy may offer potential benefits to patients with advanced invasive IPMN.