ABSTRACTPurposeEndoscopic treatment and laparoscopic surgery are minimally invasive options for early treatment of colorectal cancer, however, more evidence of the long-term outcomes between the two procedures is needed to guide clinical decisions. Therefore, this study aimed to compare the oncologic outcomes between endoscopic and laparoscopic treatment for early colorectal cancer.
MethodsThe study group included 60 patients who underwent endoscopic treatment and 38 patients who underwent laparoscopic surgery for early colorectal adenocarcinoma between January 2010 and December 2013 at a single study site.
ResultsHistopathological diagnoses showed that 43 (78.3%) carcinomas in the endoscopic submucosal dissection group were mucosal to sm1, 13 (21.7%) were sm2 or deeper, and 17 high-risk cases (28.3%) in the endoscopic group underwent additional surgery. The median operation time, time to sips of water, and length of hospital stay were significantly shorter in the endoscopic group than in the laparoscopic group. The overall survival rates of patients in the endoscopic group and laparoscopic groups were 91.5% and 87.4%, respectively (P=0.391), and the disease-free survival rates were 90.4% and 87.4% (P=0.614), respectively. Systemic recurrences occurred in two patients (1.6%) in the endoscopic group and one patient (2.0%) in the laparoscopic group. Local recurrence combined with systemic recurrence in one patient (0.8%) in the endoscopic group.
INTRODUCTIONColorectal cancer (CRC) is the third most frequently diagnosed cancer in the world although almost a quarter of the cases (23%) are stage 1 [1]. In early CRC, treatment options include endoscopic procedures and surgical resection. Patients diagnosed early with CRC and little possibility of lymph node metastasis are initially treated by endoscopic procedures such as endoscopic mucosal resection and endoscopic submucosal dissection (ESD). However, patients at a high-risk for lymph node metastasis, including those with submucosal invasion depth ≥ 1,000 μm, poorly differentiated adenocarcinoma, lymphovascular invasion, and positive lateral and/or vertical resection margins, should be surgically treated with a radical lymphadenectomy [2].
Laparoscopic colorectal resection has become an alternative standard procedure for treating colorectal diseases, including malignant tumors [3]. Several prospective randomized control trials and meta-analyses on laparoscopic surgery for CRC have demonstrated that laparoscopic surgery is superior to open surgery in terms of improved perioperative outcomes such as lesser pain, smaller incision, faster recovery of gastrointestinal function, and shorter hospital stay [4–7]. In addition, studies comparing endoscopic treatment and laparoscopic surgery for early CRC have been published. However, more data are needed regarding the long-term oncologic outcomes between these two minimally invasive treatments. Therefore, this study aimed to compare the clinical and long-term oncologic outcomes between endoscopic procedures and laparoscopic surgeries in patients who were treated for early CRC.
METHODSPatients and data collectionData on patients treated between January 2010 and December 2013 were collected from the departments of gastroenterology and colorectal surgery at the study site. In total, 60 patients who underwent an endoscopic procedure for T1 colorectal adenocarcinoma and 38 patients who underwent laparoscopic surgery for T1N0M0 colorectal adenocarcinoma were enrolled. The exclusion criteria included synchronous or previous malignancies, malignancies other than adenocarcinoma, and cancer related to familial adenomatous polyposis or hereditary nonpolyposis CRC. The study protocol was approved by the Institutional Review Board of the Keimyung University Dongsan Medical Center (IRB No. 2020-07-097) and informed consent was obtained from all patients.
Using retrospective data collection, information on patient demographics included age, sex, preoperative carcinoembryonic antigen, body mass index, and location of the tumor. The perioperative details of the endoscopic procedure included the type of procedure, depth of invasion, margin status, and morbidity after endoscopic resection. The laparoscopic surgery outcomes included the type of operation, the number of harvested lymph nodes, and morbidities after surgery. Clinicopathologic comparative outcomes included operation time, recovery, mortality, tumor stage, histology, tumor size, and lymphovascular invasion. Oncologic outcomes included overall survival (OS) rate and disease-free survival (DFS) rate and recurrence pattern.
Indication of endoscopic treatment and laparoscopic surgeryThe indications for endoscopic resection of the colorectal neoplasia in our institution are: (1) colorectal neoplastic lesion which was not suspected to be a deep submucosal invasive cancer based on white-light endoscopy and chromoendoscopy and (2) a laterally spreading tumor defined by flat neoplastic lesions that are 10 mm in size or larger and extending laterally and circumferentially, rather than vertically [8]. When the lesion was suspected to be a deep submucosal (sm2) invasive cancer based on endoscopic features such as loss of lobulation, expansive appearance, surface ulceration, surrounding convergent folds, or signs of non-lifting, endoscopic resection was not performed [9,10].
After endoscopic resection, patients with high-risk factors for recurrence chose surgical treatment or regular follow-up at a multidisciplinary clinic after endoscopic resection was done (Fig. 1).
Endoscopic techniqueEndoscopic resections were performed by three expert endoscopists who have over 8 years of experience in therapeutic endoscopy. All procedures were performed with a single-channel endoscope (GIF-H260, GIF-Q260J; Olympus Co., Tokyo, Japan) and electrosurgical unit (VIO300D; ERBE, Tübingen, Germany). According to the institutional protocol, two types of endoscopic resections were applied for early colorectal cancer (ECC) as follows: ESD alone and ESD with snaring (ESD-S). The decision to perform ESD alone or ESD-S was made by the individual endoscopist, based on the ESD guidelines for CRC [11].
For ESD, the mucosa around the tumor was incised with a dual knife (Dual Knif; Olympus Co.) at a 2–3 mm periphery from the lesion to create a tumor-free lateral resection margin after the submucosal injection (Figs. 2, 3). Most of the submucosal dissection was performed with the dual knife alone, but in some cases, a hook knife (Hook Knife; Olympus Co.) was also used. For ESD-S, resection of the remnant undissected tissue was snared when there was insufficient primary submucosal dissection.
Laparoscopic techniqueThe laparoscopic surgeries followed the general principles of complete mesocolic excision and total mesorectal excision with central vascular ligation for CRC as previously described [11]. The primary tumor was interfaced by sharp dissection between the visceral plane and the parietal fascia layer along with the entire regional mesocolon and mesorectum in an intact package. We used 5 ports: two 12-mm ports for a camera (umbilicus) and a working port (above the umbilicus, left side, midclavicular line) and another three 5-mm ports in each remaining quadrant.
Statistical analysisThe results are presented as medians with ranges for continuous outcomes, and as frequencies with percentages for categorical outcomes. Categorical variables were analyzed using chi-square and Fisher exact tests. Continuous variables were analyzed with the independent t-test and Mann-Whitney test. The Kaplan-Meier method and log-rank test were used for survival analysis. Recurrence was defined as the presence of a radiologically confirmed or histologically diagnosed tumor. The location of recurrence was defined as the first site of recurrence after complete resection. Local recurrence was defined as any tumor recurrence in the surgical field. OS was defined as the time from the date of surgery to the date of the latest follow-up visit or the date of death due to any cause. DFS was defined as the time from surgery to any type of recurrence. A P-value of<0.05 was considered to indicate statistical significance. The statistical analyses were performed with SPSS version 25.0 software (IBM Corp., Armonk, NY, USA).
RESULTSBaseline characteristicsThere was no statistical difference between the two treatment groups according to age, sex, or preoperative carcinoembryonic antigen levels. Body mass index was statistically higher in the laparoscopic group than in the endoscopic group (25.1 kg/m2 vs. 24.4 kg/m2, P=0.050) (Table 1). Tumors tended to be more frequent in the rectum of patients in the endoscopic group and in the sigmoid colon the laparoscopic group (P=0.093).
Operative outcomes of endoscopic treatmentOperative outcomes of endoscopic treatment are described in Table 2. In total, 25 patients (41.7%) underwent ESD and 35 patients (58.3%) underwent ESD-S. There were 29 (48.3%) tumors invading the muscularis mucosa and 31 (51.7%) tumors invading the submucosa. Thirteen tumors (21.7%) invaded deeper than 1 mm and 16 tumors (26.7%) involved the surgical margin. After endoscopic treatment, there were three cases with bleeding (5.0%), six cases with perforation (10.0%), and two cases with bleeding and perforation (3.3%). Among the three patients who had post-procedure bleeding, two patients underwent coagulation during the procedure and one patient was observed with conservative management. Four patients with post-procedure perforation were treated by clipping during the procedure and the others recovered after conservative management. Among the two patients who had bleeding and perforation simultaneously during the endoscopic procedure, one patient was treated using hemo-clipping and the other patient was converted to the surgical treatment.
Operative outcomes of laparoscopic surgeryOperative outcomes of laparoscopic surgery are provided in Table 3. The most frequently performed procedure was 13 right hemicolectomies (32.2%) and the median number of retrieved lymph nodes was 19 (range, 3–49). Six patients had morbidities within 30 days after surgery including anastomotic site bleeding, chyle leakage, ileus, pseudomembranous colitis, difficulty voiding, and wound infection. One patient who had anastomotic site bleeding was treated with argon plasma coagulation via sigmoidoscopy. The patient with chyle leakage and ileus was managed with conservative management. There were no severe complications that required reoperation and no conversion to open surgery occurred.
Clinicopathologic outcomesThe clinicopathologic outcomes of the two treatment groups are summarized in Table 4. The median operation time was significantly longer for the laparoscopic group (187.5 minutes vs. 35.8 minutes, P<0.001). Time to sips of water and length of hospital stay were significantly shorter in the endoscopic group compared to the laparoscopic group (5 days vs. 1 day, P<0.001; 7 days vs. 2 days, p<0.001, respectively). The mean tumor size was larger in the endoscopic group compared to the laparoscopic group (1.5 cm vs. 1.8 cm, P=0.016) and the ratio of moderate and poorly differentiated lesions was significantly higher in the laparoscopic group compared to the endoscopic group (P=0.018). There was no significant difference between tumor stage or lymphovascular invasion between the two groups. Two patients who were treated for T1 cancer were finally diagnosed as stages T3 and T4 after endoscopic resection.
Oncologic outcomes and recurrence patternsThe median follow-up period was 56 months in the laparoscopic group and 50 months in the endoscopic group (Table 5). The OS rate was not significantly different between groups (87.4% in the laparoscopic group and 91.5% in the endoscopic group, P=0.391) (Fig. 4A). The DFS rates were 87.4% in the laparoscopic group and 90.4% in the endoscopic group (P=0.614) (Fig. 4B). There was no local recurrence in either group. Table 6 summarizes the cases of four patients with recurrence according to treatment type. There were three systemic recurrences in the liver (laparoscopic group: n=1 [2.6%], endoscopic group: n=2 [3.3%]). One rectal cancer patient who underwent an endoscopic procedure had local recurrence and systemic recurrence in the liver and lungs (0.8%).
After endoscopic treatment for early CRC, 32 patients had more than one high-risk factor (Fig. 1). After a multidisciplinary team approach, 17 patients (28.3%) underwent additional surgery due to high-risk factors and 15 patients did not undergo additional surgery due to old age and/or severe co-morbidities. Table 7 shows the details from patients who did not undergo additional surgery despite high-risk factors after endoscopic resection. Two patients were treated with chemoradiotherapy or chemotherapy and three patients underwent repeated endoscopic resection. Three patients were treated with repeated endoscopic procedures including ESD or ESD-S and 10 patients had regular follow-up without further treatment. Among the patients who underwent endoscopic re-resection, there was one systemic recurrence in the lungs 1 year after ESD.
DISCUSSIONThis study aimed to compare endoscopic and laparoscopic outcomes for early CRC treatment to clarify clinical decision-making. Results of the present study show that patients who underwent endoscopic treatment had superior short-term clinical outcomes, including shorter operating time and hospital stay, and similar long-term oncologic outcomes compared to those who underwent laparoscopic surgery. Therefore, we recommend an endoscopic treatment as an initial intervention for early CRC with multidisciplinary approach.
As endoscopic techniques develop, there are attempts to expand the indications for this approach, including ESD, to treat early CRC with various techniques [12]. However, more evidence is needed regarding the short-term clinical outcomes between endoscopic and laparoscopic interventions for treating early CRC and comparative studies with long-term oncologic outcomes between the two groups are lacking [13,14]. Kiriyama et al. [14] reported that en bloc and curative resection rates for endoscopic and laparoscopic interventions were 87% and 80%, respectively. In the same study, the rate of post-procedure morbidities, including perforations and bleeding after endoscopic procedures, was 6.4% and in the laparoscopic group, the mean operation time was 206 minutes and the rate of complications including surgical site infection, pelvic abscesses, anastomotic leakages, and anastomotic bleeding was 12.6%. Inoue et al. [13] demonstrated that en bloc and curative resection rates were 93.7% and 87.4%, respectively, for endoscopic and laparoscopic interventions and complication rates were 8.4% during 5 days of hospital stay in the ESD group. In the laparoscopic surgery group, operative times were 228 minutes and the average hospital stay was 9 days with a 5.4% postoperative complication rate. In the present study, the operation time, time to sips of water, and hospital stay were shorter in the endoscopic group compared to the laparoscopic group with similar complication rates. These data support previous studies that demonstrate the feasibility and minimally invasive nature of endoscopic resection.
In a multicenter study that demonstrated the clinical and long-term outcomes of endoscopic treatment reported that the DFS and recurrence rates of early CRC in patients with low-risk factors, such as well to moderately differentiated, ≥ 2 mm cancer-free margin, and Haggitt invasion level 1–3, were 98% and 0.8%, respectively, whereas the rates in patients with high-risk factors were 89% and 6.6%, respectively [15]. Ikematsu et al. [16] also reported that overall recurrence-free survival rates and recurrence rates were 96% and 0% for patients with colon cancer and 90% and 6.3% for rectal cancer, respectively. In a high-risk group, recurrence-free survival rates and recurrence rates were 96% and 1.4% for colon cancer and 77% and 16.2% for rectal cancer patients, respectively [16]. In the present study, the DFS and recurrence rates were 90.4% and 4.1%, respectively, in the endoscopic group. Among the patients in this group, 32 patients (53.3%) were identified as high-risk and 17 of these patients were referred to the surgical department for radical resection. As a result, there was no recurrence in these patients, providing evidence for the long-term oncologic safety of endoscopic resection with salvage surgery for ECC.
The 15 patients who refused a radical resection despite a recommendation in the present study, cited old age and co-morbidities as the reason. Among these patients, five patients were treated with chemotherapy, chemoradiation, or endoscopic re-resection. There was one systemic recurrence in the lungs one year after re-ESD. The tumor was a moderately differentiated tubular adenocarcinoma with submucosal 3 mm invasion and suspected angiolymphatic invasion. Based on our study, surgical resection could be recommended after the identification of high-risk factors after endoscopic treatment, although careful observation of patients with co-morbidities and/or old age is also recommended.
Several multicenter prospective studies have demonstrated the feasibility and safety of laparoscopic surgery for CRC and this surgical approach has become the technique of choice for performing CRC resection [17,18]. In the present study, short-term outcomes of laparoscopic surgery including the time to oral feeding, length of hospital stay, and operation time were comparable to previous studies. Regarding the long-term outcomes, the one study reported that the 5-year survival rates were about 80% and recurrence rates were 8% for stage I CRC in laparoscopic groups [17]. In the present study, the OS rates were 87.4% and recurrence rates were 2.6% in the laparoscopic patients, demonstrating the long-term oncologic safety of laparoscopic surgery.
There are some limitations to the current study including its retrospective nature, selection bias, small sample size, and single-center study. However, this study adds to the literature by providing evidence for the evaluation of long-term oncologic outcomes between two minimally invasive treatments.
In conclusion, endoscopic resection for early CRC can be performed safely with better short-term clinical outcomes and similar long-term oncological outcomes compared to laparoscopic surgery. We recommend an endoscopic treatment as an initial intervention for early CRC with multidisciplinary approach.
CONFLICT OF INTERESTCONFLICT OF INTEREST
Sung Uk Bae is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
ACKNOWLEDGMENTSThis work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No. 2021R1F1A1064310).
REFERENCES1. Cronin KA, Lake AJ, Scott S, Sherman RL, Noone AM, Howlader N, et al. Annual report to the nation on the status of cancer, part I: national cancer statistics. Cancer 2018;124:2785-800.
2. Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, et al. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc 2015;27:417-34.
3. Neki K, Eto K, Kosuge M, Ohkuma M, Noaki R, Hashizume R, et al. Comparison of postoperative outcomes between laparoscopic and open surgery for colorectal cancer. Anticancer Res 2017;37:5173-7.
4. Biondi A, Grosso G, Mistretta A, Marventano S, Toscano C, Gruttadauria S, et al. Laparoscopic-assisted versus open surgery for colorectal cancer: short- and long-term outcomes comparison. J Laparoendosc Adv Surg Tech A 2013;23:1-7.
5. Karanikolic A, Golubovic I, Radojkovic M, Pavlovic M, Sokolovic D, Kovacevic P. Comparison of recurrence patterns of colorectal cancer in laparoscopic and open surgery groups of patients: a meta-analysis. J BUON 2018;23:302-11.
6. Tong G, Zhang G, Liu J, Zheng Z, Chen Y, Cui E. A meta-analysis of short-term outcome of laparoscopic surgery versus conventional open surgery on colorectal carcinoma. Medicine (Baltimore) 2017;96:e8957.
8. Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 2006;55:1592-7.
9. Kakushima N. Endoscopic diagnosis for the depth of early colorectal cancer. J Gastroenterol Hepatol 2010;25:850-1.
10. Park W, Kim B, Park SJ, Cheon JH, Kim TI, Kim WH, et al. Conventional endoscopic features are not sufficient to differentiate small, early colorectal cancer. World J Gastroenterol 2014;20:6586-93.
11. Kim NK, Kim YW, Han YD, Cho MS, Hur H, Min BS, et al. Complete mesocolic excision and central vascular ligation for colon cancer: principle, anatomy, surgical technique, and outcomes. Surg Oncol 2016;25:252-62.
12. Nishizawa T, Yahagi N. Endoscopic mucosal resection and endoscopic submucosal dissection: technique and new directions. Curr Opin Gastroenterol 2017;33:315-9.
13. Inoue T, Koyama F, Kuge H, Ueda T, Obara S, Nakamoto T, et al. Short-term outcomes of endoscopic submucosal dissection versus laparoscopic surgery for colorectal neoplasms: an observational study. J Anus Rectum Colon 2018;2:97-102.
14. Kiriyama S, Saito Y, Yamamoto S, Soetikno R, Matsuda T, Nakajima T, et al. Comparison of endoscopic submucosal dissection with laparoscopic-assisted colorectal surgery for early-stage colorectal cancer: a retrospective analysis. Endoscopy 2012;44:1024-30.
15. Yoda Y, Ikematsu H, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. A large-scale multicenter study of long-term outcomes after endoscopic resection for submucosal invasive colorectal cancer. Endoscopy 2013;45:718-24.
16. Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, et al. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology 2013;144:551-9.
Fig. 2Representative pictures for of endoscopic resection for early colorectal cancer: endoscopic submucosal dissection. (A) A laterally spreading tumor approximately 3.5×3.5 cm is identified. (B) A submucosal injection is performed. (C) A mucosal incision and a submucosal dissection are performed. (D) Subsequent submucosal dissection is performed. (E) After submucosal dissection is complete, a clear ulcer is created. (F) The specimen is resected en bloc. 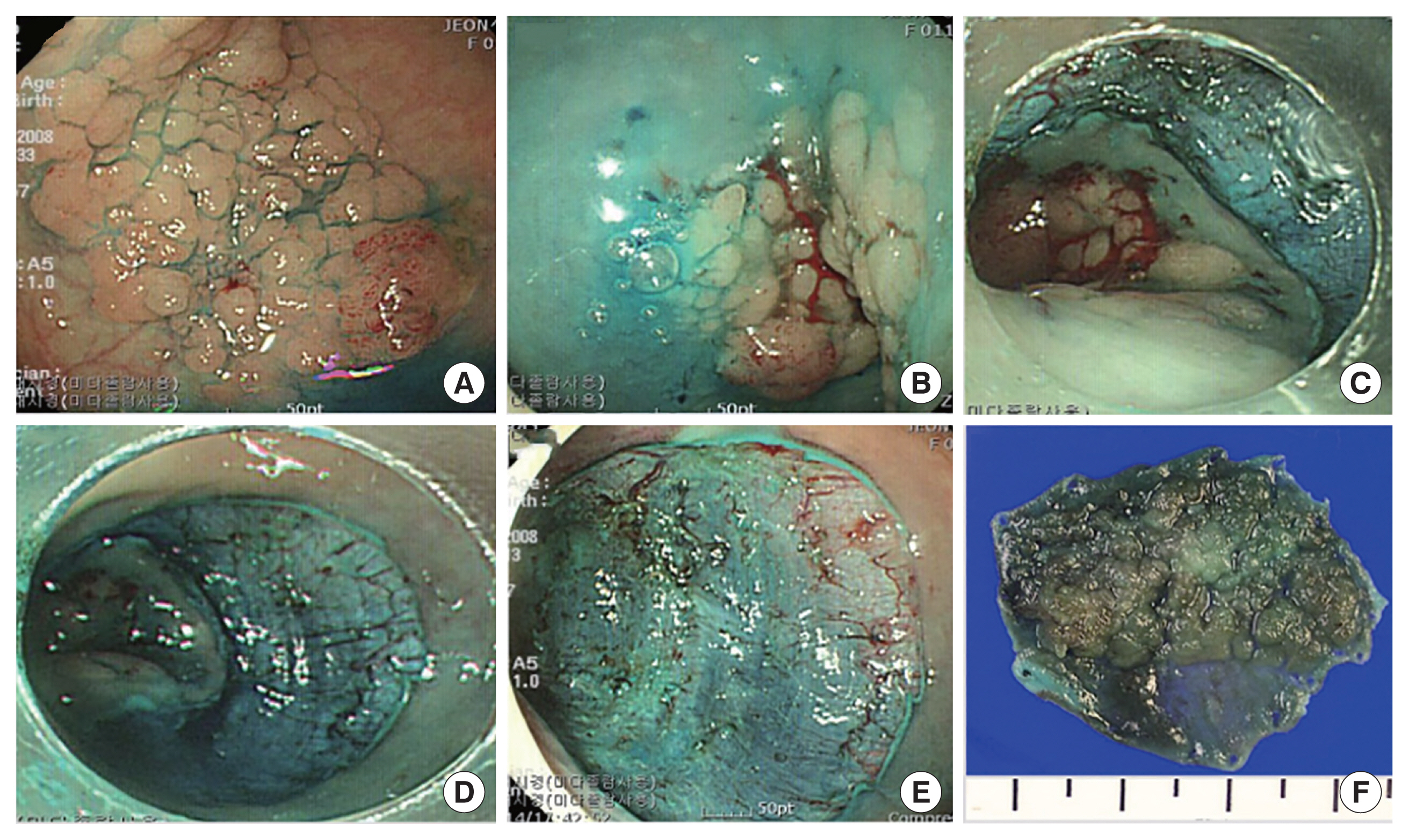
Fig. 3Representative pictures for of endoscopic resection for early colorectal cancer: endoscopic submucosal dissection with snaring. (A) A laterally spreading tumor approximately 3×2.5 cm is identified. (B) A submucosal injection and a mucosal incision are performed. (C) After half of the lesion is dissected with the ESD method, the remnant lesion was resected by snaring. (D) The snaring resection at the final stage is performed. (E) After snaring resection, a clear ulcer is created. (F) The specimen is resected en bloc. 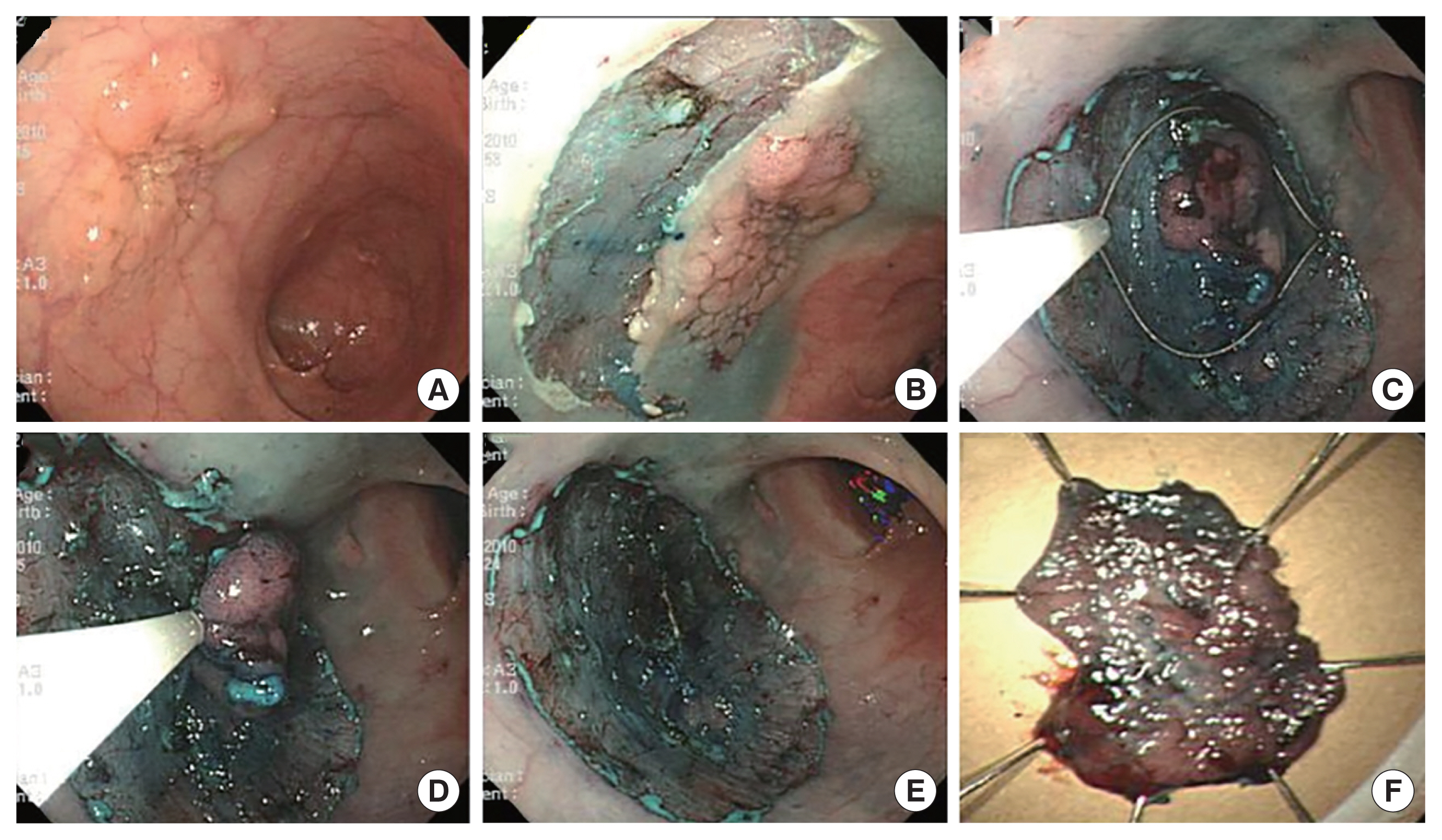
Fig. 4Five-year overall rate (A) and disease-free survival rate (B) in the endoscopic and laparoscopic groups. 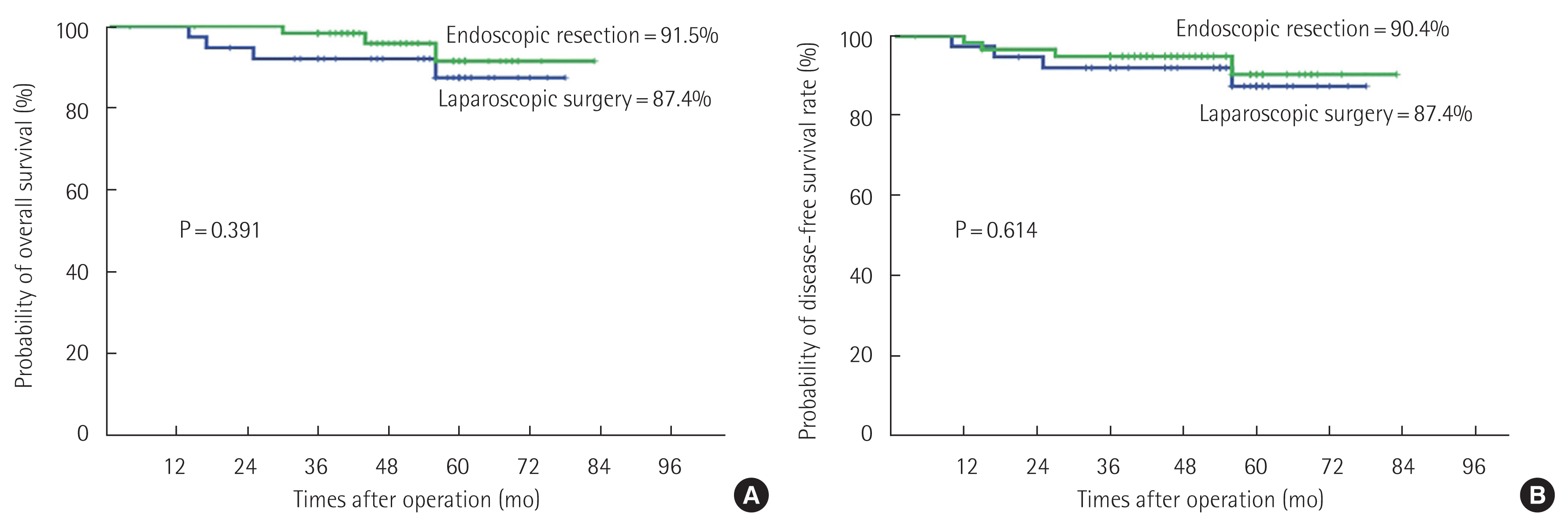
Table 1Baseline patient and tumor characteristics Table 2Endoscopic treatment outcomes Table 3Laparoscopic surgery outcomes Table 4Clinicopathologic outcomes of laparoscopic surgery and endoscopic treatment Table 5Oncologic outcomes and recurrence patterns for laparoscopic surgery and endoscopic treatment Table 6Summary of four cases of recurrence after endoscopic procedure or surgical treatment for early colorectal cancer Table 7Patients with high-risk factors who did not undergo additional surgery after endoscopic resection
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||