INTRODUCTION
Colorectal cancer is one of the most common cancers worldwide, particularly in the economically developed world [1]. Recent developments in the diagnosis of colorectal cancer have led to an increase in the detection of early-stage cancer, especially pathologically T1. Patients with T1 colorectal cancer have a good prognosis, and the 5-year recurrence rate has been reported to be 1.3% after radical surgery [2].
The liver is the first and most common site of distant metastasis from colorectal cancer, involving up to 70% of patients and is the major cause of death [3–5]. The incidence of synchronous liver metastases in colorectal cancer has been reported to be about 30% to 40% [4]. But, T1 colorectal cancer with synchronous liver metastasis is considered to be rare [6]. The form of metastasis from T1 colorectal cancer is mainly lymph node metastasis, not hematogenous metastasis like liver metastasis [7,8]. We report a rare case of T1N0 colon cancer without lymphovascular invasion with synchronous liver metastasis not detected by preoperative imaging study. Written informed consent was obtained.
CASE REPORT
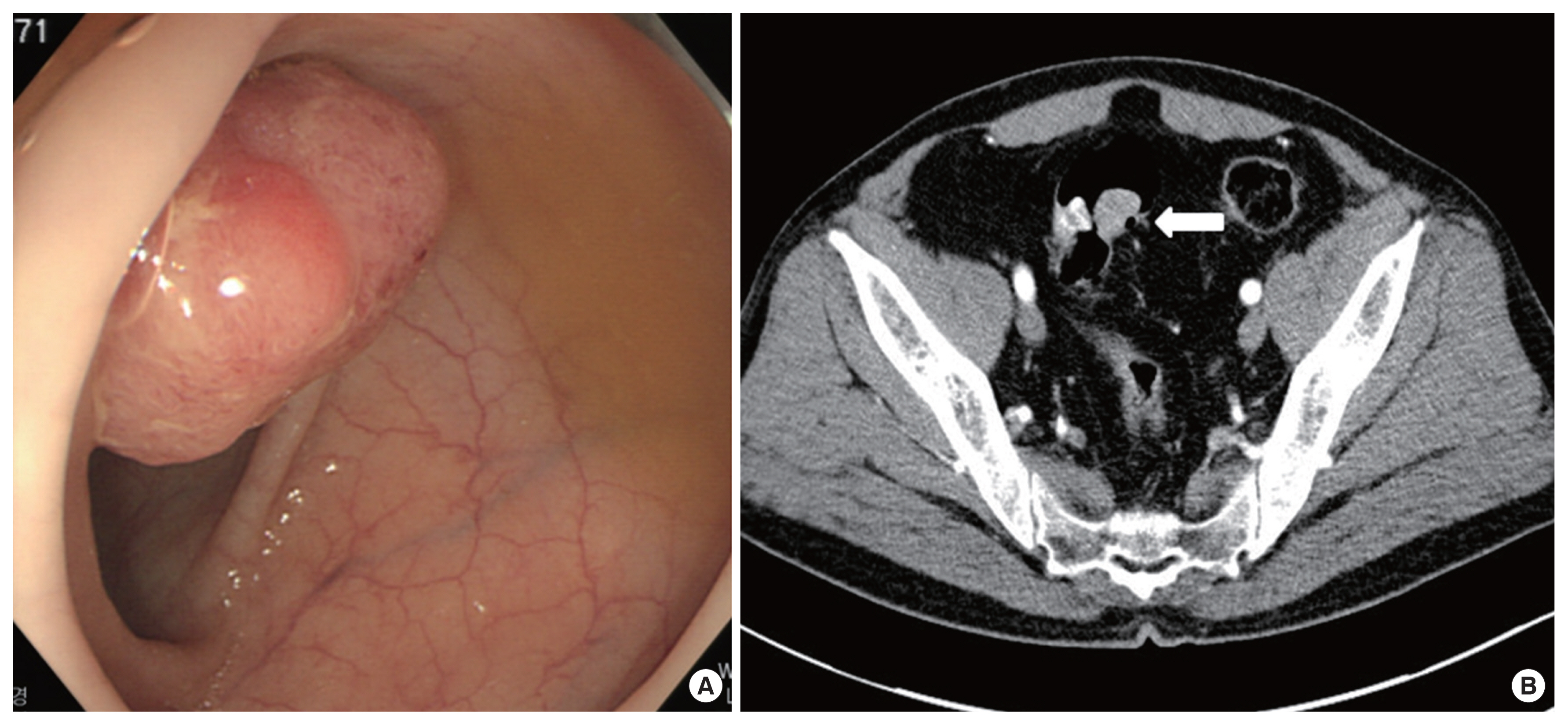
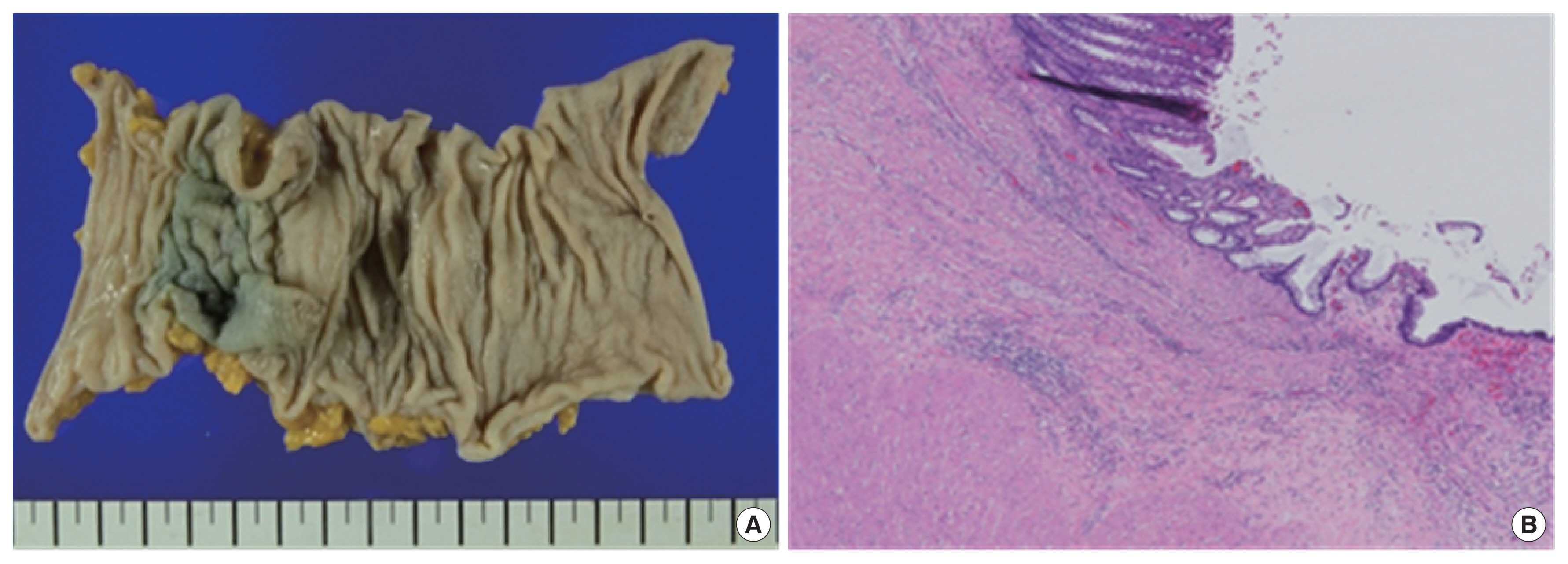
A 54-year-old man presented to our department for treatment of sigmoid colon cancer following an endoscopic submucosal dissection (ESD). He had no specific personal or family history of malignancy. He received colonoscopy and abdominal computed tomography (CT) scan in December 2013 because of blood tinged stool. Several polyps were found and a 3-cm sized pedunculated mass was found at sigmoid colon on colonoscopy (Fig. 1A). Abdominal CT revealed an intraluminal mass at sigmoid colon, and others were unremarkable (Fig. 1B). ESD and several polypectomies were done. Microscopically, the pedunculated mass from the sigmoid colon revealed a moderately differentiated adenocarcinoma arising from a tubulovillous adenoma. The depth of submucosal invasion was 2,000 μm from the muscularis mucosa and lymphovascular invasion was not identified. The resection margin was not involved (Fig. 2). The other polyps were adenoma with low grade dysplasia. His carcinoembryonic antigen (CEA) level was 1.4 ng/mL (normal level: <1.5 ng/mL). He underwent FDG-positron emission tomography/CT (FDG-PET/CT) and there was no evidence of distant metastasis.
We performed a laparoscopic anterior resection with lymph node dissection 3 weeks after ESD. The final pathologic report indicated that there was no residual tumor and none of the 23 lymph nodes were confirmed as metastasis. There was no lymphatic, venous, or perineural invasion and the resection margins were clear (Fig. 3). His pathological stage was T1N0M0, stage I (American Joint Committee on Cancer 8th).
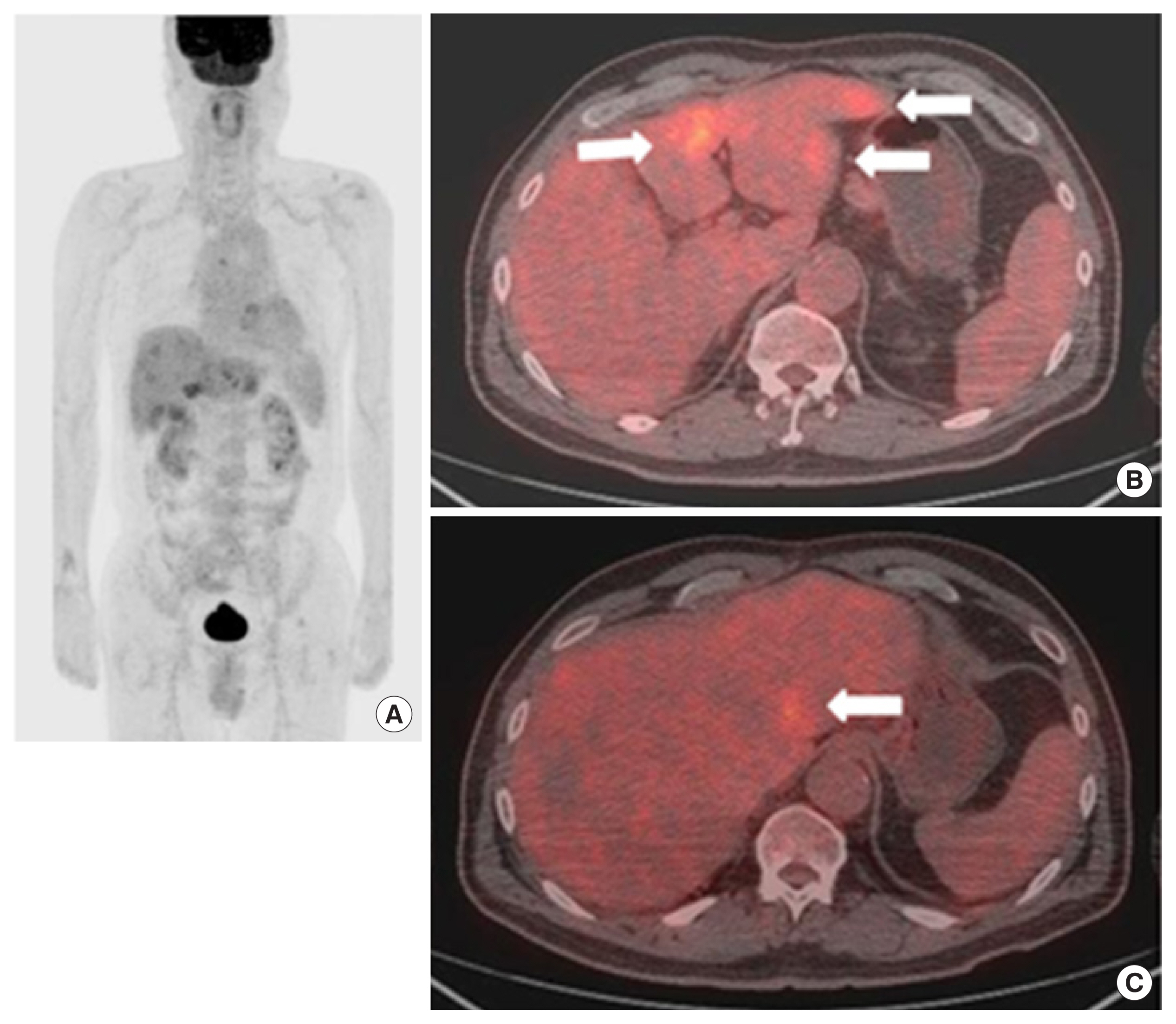
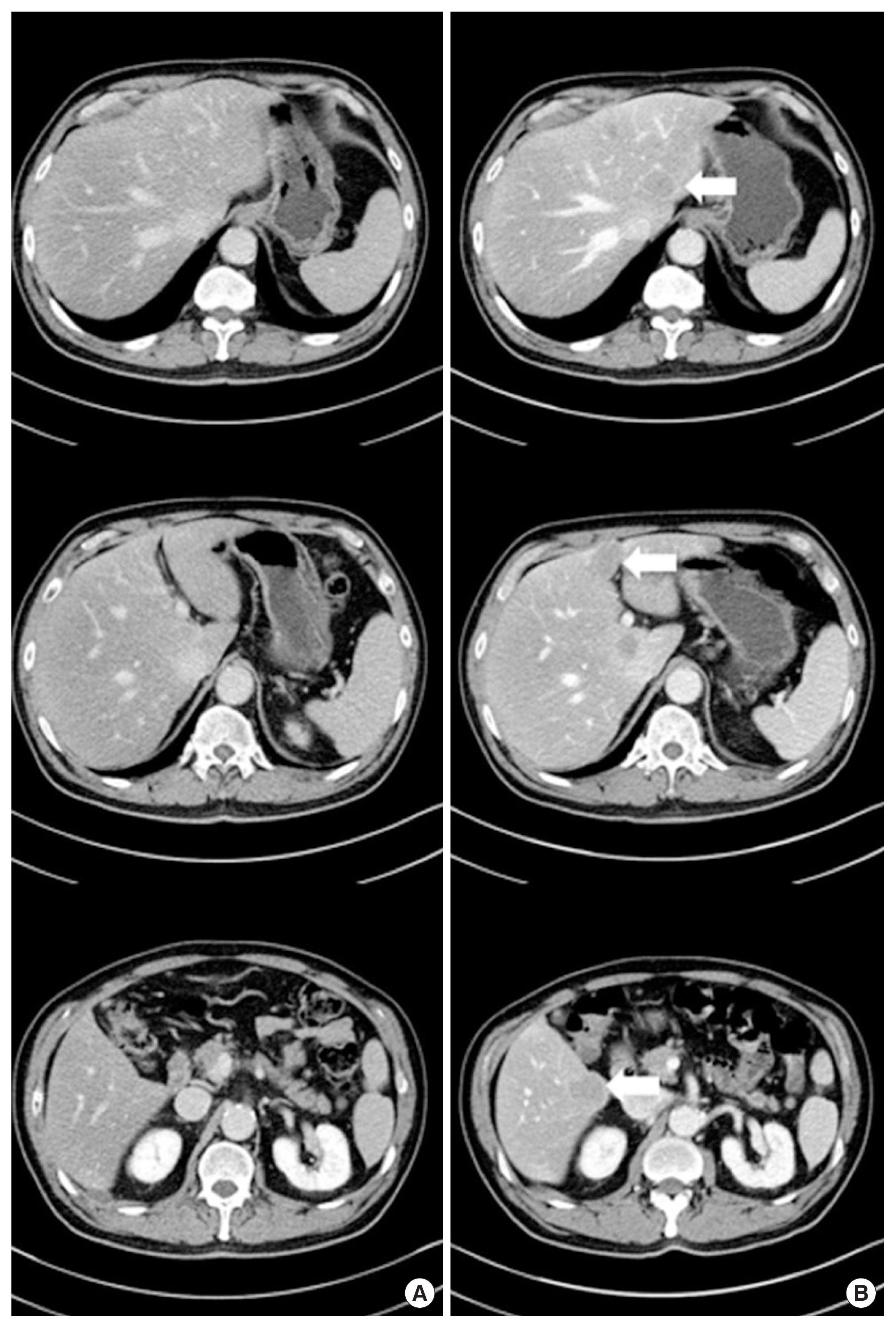
After 3 months, we checked routinely the patient’s CEA level and his CEA level was elevated (7.26 ng/mL, normal level: <1.5 ng/mL). Then, he underwent abdominal CT and FDG-PET/CT. The abdominal CT and FDG-PET/CT showed multiple metastases in both hepatic lobes (SUVmax: 5.6) without evidence of local recurrence or lymphadenopathy (Figs. 4, 5). We strongly suspected a synchronous liver metastasis not detected by imaging study as opposed to a systemic recurrence. Surgery was impossible, because the finding was multiple hepatic metastases. Our plan was palliative, targeted-agent chemotherapy. But, unfortunately the patient has elected chemotherapy in another hospital, and the progress since then is unknown.
DISCUSSION
T1 colorectal cancer is considered to have a good prognosis, and the recurrence rate has been reported to be 1.3% after radical surgery [2]. The form of metastasis in T1 colorectal cancer is mainly lymph node metastasis, but some patients exhibit liver metastasis. A multicenter study in Japan reported that 153 (8.5%) of 1,806 patients with T1 colorectal cancer had lymph node metastases, but 22 (1.2%) of them had liver metastases, and three of them (0.2%) had synchronous liver metastases. At the point of synchronous or metachronous distant metastases from T1 colorectal cancer, lymph node metastases, depth of invasion and venous invasion are thought to be the risk factors. Okano et al. [6] reported that seven of 213 patients (3.3%) with T1 colorectal cancer without lymph node metastasis had liver metastases and two (0.2%) of them had synchronous liver metastases. Okano et al. [6] reported that venous invasion and the grade of differentiation of the tumor are closely related to liver metastases in T1 colorectal cancer without lymph node metastasis. In our case, the final pathologic report indicated that the mass was moderately differentiated adenocarcinoma with 2,000 μm depth of submucosal invasion but there was no lymphatic or venous invasion. Submucosal invasion and grade of differentiation were thought as risk factors for liver metastases.
Synchronously detected metastases were defined as metastases detected prior to or during resection of the primary tumor, and in the case of non-resected patients as detected prior to or concurrently with the primary tumor. There were no metastases in the preoperative evaluation. However, less than 6 months later, multiple liver metastases were detected in the abdomen CT, PET/CT. Usually, it is reasonable to assume rapid progressive synchronous liver metastases rather than metachronous liver metastases.
Liver resection is the only potentially curative treatment for colorectal liver metastasis and in selected groups, the 5-year median survival has been reported to be up to 30% (range, 15%–67%) [9]. If left untreated, virtually all patients with liver metastases would be dead within 5 years with a median survival of 8 months [10]. In such case, preoperative evaluation of liver metastasis and prediction of metastasis is very important on patient selection to avoid inappropriate treatment. A multi-modality strategy is recommended since no single modality can accurately detect all colorectal liver metastases [11].
In preparing this case report, we looked closely at the preoperative evaluation conducted in collaboration with radiology and nuclear medicine. There was a high possibility of liver metastatic lesions at the time of preoperative evaluation, but the review did not reveal any suspected lesions. Although this is a rare case, many studies are needed to find such rapid synchronous metastases that are not detected in current and routine CT and PET/CT.
Magnetic resonance imaging (MRI) is particularly useful in characterizing indeterminate lesions detected on other modalities such as CT or unenhanced ultrasonography; it can also identify some lesions not visualized on either of these modalities [12]. Cho et al. [13]. reported that 121 equivocal hepatic lesions were detected on preoperative abdominal CT in 65 out of 494 patients (13.2%) who were diagnosed with colorectal cancer, and 11 lesions were classified as definitive metastatic lesion based on subsequent liver MRI. A prospective randomized trial [14] reported that 35 patients who were diagnosed with rectal cancer were divided into two groups, abdominal CT group (15 patients) and diffusion weighted liver MRI group (DWMR; 20 patients). The per-patient sensitivity/specificity was 50%/100% for CT, and 100%/94% and 100%/100% for reader 1 and 2 for DWMR. The per-lesion sensitivity of CT and DWMR were 17% and 89%, respectively. In a meta-analysis, on a per-patient basis, FDG-PET had significantly higher sensitivity compared with that of the other modalities in the detection of colorectal liver metastasis. Yet, on a per-lesion basis, sensitivity estimates for MRI with contrast agent were significantly superior to those for helical CT with 45 g of iodine or less [4]. In our case, we did not take liver MRI by conventional preoperative evaluation protocol (National Comprehensive Cancer Network Guideline, 2019) and we could not diagnose liver metastases on preoperative abdominal CT and FDG-PET/CT. We believe that preoperative liver MRI with contrast agent can be useful in selected patients with liver metastases not visualized on abdominal CT.
According to the NCCN guideline 2019, stage 1 colon cancer requires more careful surveillance in stage 1 colon cancer, since CEA or other specific imaging studies are not included in postoperative follow-up guidelines. Besides image study, investigating metastasis associated in colon cancer 1 (MACC1) and MET expression in colorectal adenoma, Tis, early-stage invasive (T1 and T2), and advanced adenocarcinoma with liver metastasis can be done using immunohistochemistry. Stepwise elevation of MACC1 expression in key points of colorectal cancer development suggests that MACC1 may contribute to cancer initiation and early invasive growth. High expression of both MACC1 and MET may relate to distant metastasis [15]. The possibility of metastasis and prognosis of colorectal cancer through MACC1 may be helpful in increasing the survival rate of patients by prompt and appropriate testing. Unfortunately, we did not routinely test MACC1 and MET oncogene, so the above tests could not be performed in these patients. As such, there is regret that more appropriate tests and treatments would have been faster.
In conclusion, there are few well-organized reports about T1 colorectal cancer with synchronous liver metastases, because T1 colorectal cancer with synchronous liver metastasis is very rare. If observations of this disease pattern increase, it will be possible to assess the risk factor of liver metastases in T1 colorectal cancer, and protocols for preoperative evaluation should be changed to allow more accurate imaging of liver metastases. Even with T1 colorectal cancer, it is possible to predict the prognosis and the possibility of metastasis through genetic studies, and to provide patients with faster and more appropriate treatments if appropriate image studies such as liver MRI are considered.