INTRODUCTION
Carcinoembryonic antigen (CEA) is one of the most extensively used clinical tumor markers in cases of colorectal cancer (CRC). The primary use of serum CEA levels as a tumor marker is in post-surgical surveillance of CRC to detect distant metastasis or tumor recurrence [1]. Serial CEA measurements can detect recurrent CRC with a sensitivity of between 60% and 95%, and a specificity of between 34% and 91% [2,3]. Thus, monitoring CEA levels after treatment provides an early indication and allows for secondary surgery or other treatment modalities. In addition, serum CEA levels can be used as prognostic indicators. A high preoperative CEA level in CRC is associated with poor 5-year overall survival, while a low preoperative level is associated with good survival [4,5].
However, there are limitations (false positive and false negative test results) to the use of serum CEA as a tumor marker. Elevated serum CEA is detected in cigarette smokers, patients with benign neoplasm, and in 15% to 20% of subjects with inflammatory disorders such as ulcerative colitis, Crohn’s disease, pancreatitis, liver disease, and pulmonary infections. Thus, elevated plasma CEA levels are not specific for cancer, although very high concentrations (> 20 ng/mL) are highly suggestive of malignancy [6,7]. In case of a false negative result, serum CEA level varies by tumor grade, liver status, and the ploidy status of the tumor. For example, the mean CEA concentration in a poorly differentiated colorectal neoplasm is lower than in a well-differentiated or moderately differentiated tumor, and the CEA level of tumors with a near-diploid pattern is lower than in tumors with aneuploidy CRC [2]. These examples indicate limitations of the use of CEA as a prognostic indicator.
In colon cancer, the bloodstream first carries the cancer cells to the superior/inferior mesenteric veins (SMV/IMV), the portal vein, and then the liver, where the vessels divide into capillaries. Thus, for colorectal cancers, the SMV/IMV and portal vein act as drainage veins (DVs). Previous studies showed that the elevation of CEA level was correlated with Dukes’ staging, lymphovascular invasion, and infiltration of cancer cells in immunohistochemical staining analysis, and that the DV blood CEA (dCEA) level was more elevated than the peripheral CEA (pCEA) level [8,9]. These findings suggest that the route of CEA transfer from the tumor to the peripheral blood could be an important contributing factor to the control of peripheral CEA level, and that CEA from a DV might be more sensitive to histopathologic tumor characteristics.
On the basis of previous studies, we can hypothesize that the CEA level from the DV (SMV or IMV) could more accurately reflect histopathologic tumor characteristics than from the peripheral vein (PV) because the CEA is cleared from circulation by the liver, and that the CEA from the DV might be more strongly correlated with patients’ oncologic outcomes. The aim of this study was to explore whether the CEA level measured directly from the tumor DV can predict prognosis more accurately than that from the PV by increasing the sensitivity of CEA detection.
METHODS
Ethics statement
This investigation has been conducted in accordance with the ethical standards, the Declaration of Helsinki, and national and international guidelines. The present study was approved by the Institutional Review Board of the Severance Hospital (IRB no. 4-2017-0157).
Patients
A total of 52 patients who received curative resection between 2004 and 2005 at Yonsei University Severance Hospital, Seoul, Korea for pathologically confirmed adenocarcinoma originating in the colon were enrolled. Patients who had metastasis, palliative resection, previous cancer treatment, or no available paired blood samples from both the tumor DV and the PV were excluded.
Blood samples and ELISA
Blood samples (5 mL) were drawn from the PV and tumor DV and placed in plain tubes. PV blood was obtained 1 hour prior to surgical incision. DV blood was obtained prior to the ligation of any branch of the SMV in patients with proximal colon cancer and prior to ligation of the IMV in patients with distal colon cancers. Samples were immediately centrifuged, and plasma and serum were separately stored at −70°C until analysis. enzyme-linked immunosorbent assay (ELISA) was performed using commercially available kits (IBL-Hamburg GmbH, Hamburg, Germany) according to manufacturer’s instructions. Diluted serum was transferred to wells of plates pre-coated with primary antibody. Following the recommended incubation period, plates were washed with buffer solution, and substrate solution was added and incubated as instructed. The wells were developed with a color-reagent. Stop solution was added to each well after incubation and the optical density was measured at a wavelength of 450 nm using automated optical densitometry. Each sample was run in triplicate, and the mean value was used for analysis. If the R2 values of the standard solutions was < 0.98, data from the plate were excluded.
Statistical analysis
Statistical analysis was performed by using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Analyzed clinical parameters included age, gender, tumor node metastasis (TNM) stage, histological grade, and lymphovascular invasion. Data on molecular factor levels are shown as the mean± standard deviation. The Mann-Whitney U test, Fisher’s exact test, and McNemar test were used to compare differences in molecular factor levels between the clinical and pathological features. A Cox proportional hazards analysis was used to evaluate the relationship between pathological features, plasma molecular factor levels, and overall survival. A P-value < 0.05 was considered significant.
RESULTS
Patient characteristics
A total of 52 patients were enrolled in this study. The mean age was 60 years; approximately half of patients had stage 3 disease (Table 1). The mean pCEA and dCEA levels were 14.4± 32.0 ng/mL and 65.2± 144.7 ng/mL (P= 0.001), respectively. There was a moderate correlation between pCEA and dCEA levels (Pearson correlation coefficient: 0.6736).
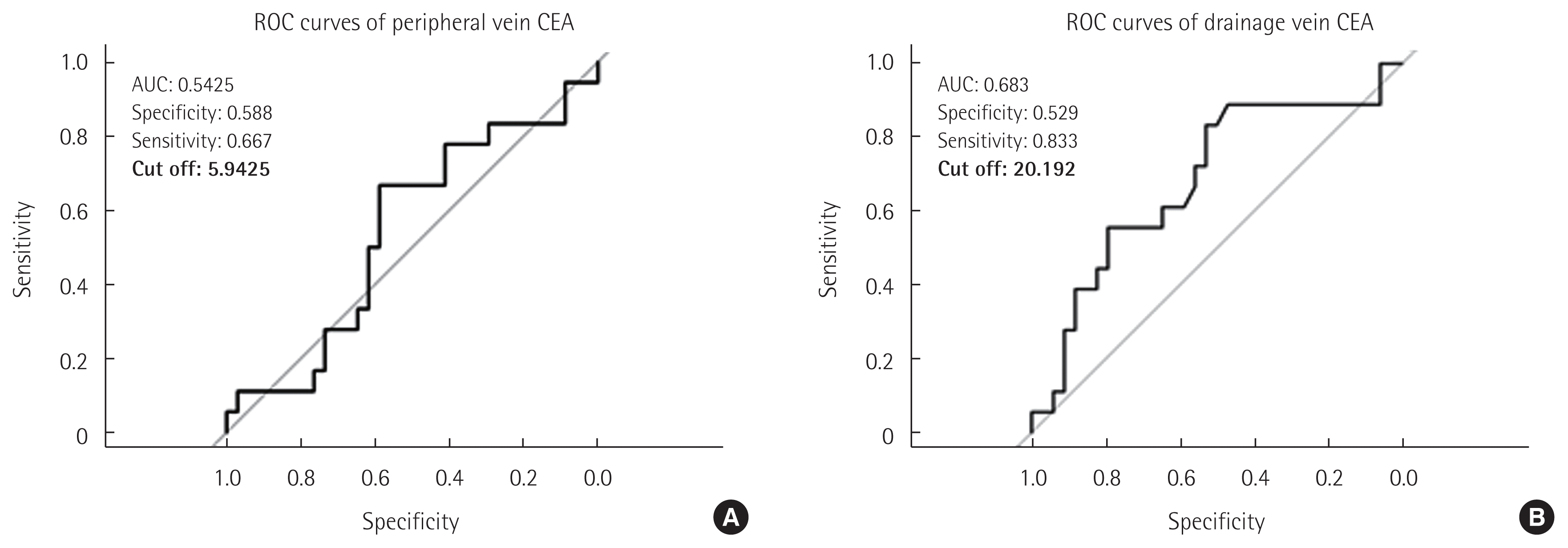
Patients were then divided into 2 groups according to whether their peripheral CEA exceeded the reference value of 5.9425 ng/mL (Fig. 1A). Clinicopathologic variables were compared; none was significantly different (Table 1). The dCEA levels in each group were 31.8± 31.9 ng/mL and 93.8± 192.0 ng/mL, respectively, but were not statistically different (P= 0.103).
Oncologic outcomes and CEA levels
To determine cut-off values for pCEA and dCEA levels, receiver operating characteristic (ROC) curves were analyzed. Through ROC curve analysis, a point most correlated to recurrence with the highest sensitivity and specificity was calculated for both pCEA and dCEA levels. For pCEA, it was determined to be 5.9425 ng/mL, the same as the reference value, for which sensitivity and specificity were 0.667 and 0.588, respectively (Fig. 1A). For dCEA, the point was 20.192 ng/mL; sensitivity and specificity were 0.833 and 0.529, respectively (Fig. 1B).
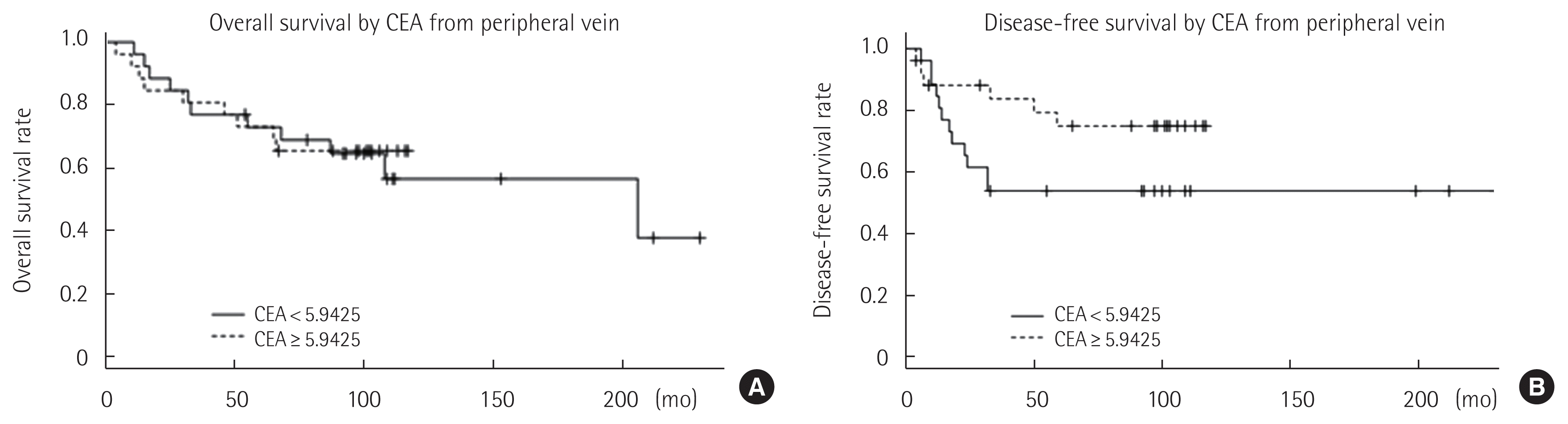
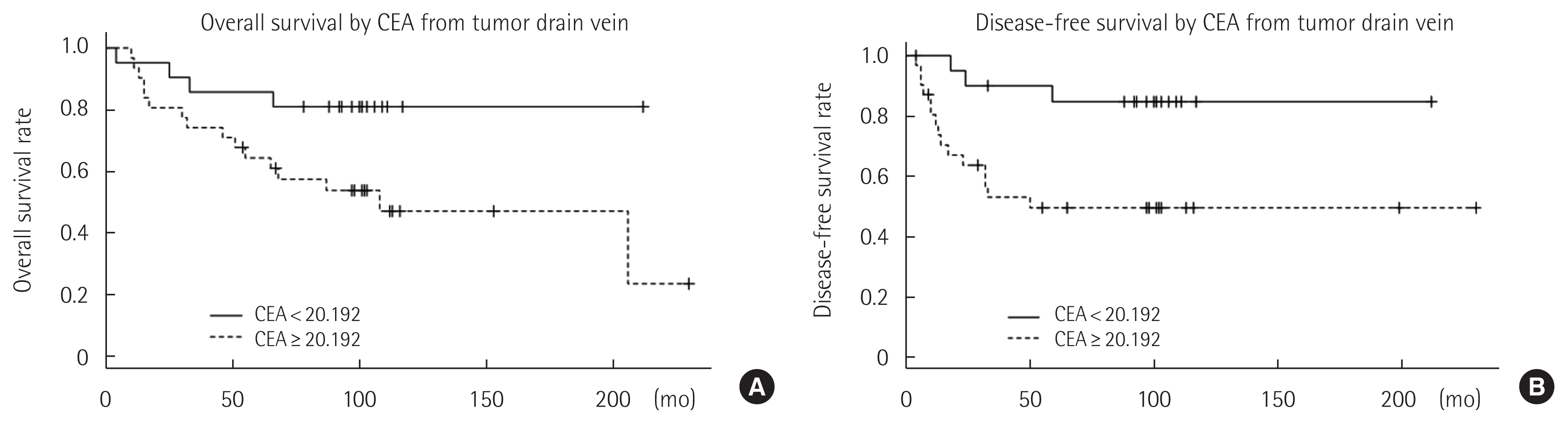
For given cut-off values of pCEA and dCEA, Kaplan-Meier survival analyses with log rank tests were performed. When the analysis was performed according to a pCEA level below 5.9425 ng/mL, both disease-free and overall survival curves were not significantly different (Fig. 2). However, patients with dCEA below 20.192 ng/mL showed better disease-free (P = 0.009) and overall survival (P= 0.033) curves than those with dCEA = 20.192 ng/mL (Fig. 3).
The Cox proportional hazard model analysis revealed the dCEA level to be a significant prognostic factor for disease-free (hazard ratio [HR]=399; 95% confidence interval [CI], 16.4–9,747; P<0.001) and overall survival (HR= 9.39; 95% CI, 1.29–68.006; P= 0.026) (Table 2). For disease-free survival, significant poor prognostic factors other than high dCEA level included a poorly differentiated tumor, complications, a Clavien-Dindo grade = 3, patients > 60 years of age, TNM stage 3 or 4, and intraoperative blood transfusion. For overall survival, factors included poorly differentiated tumors, complications, a Clavien-Dindo grade = 3, or a lymph node harvest below 12.
Significance of dCEA levels in patients with normal pCEA
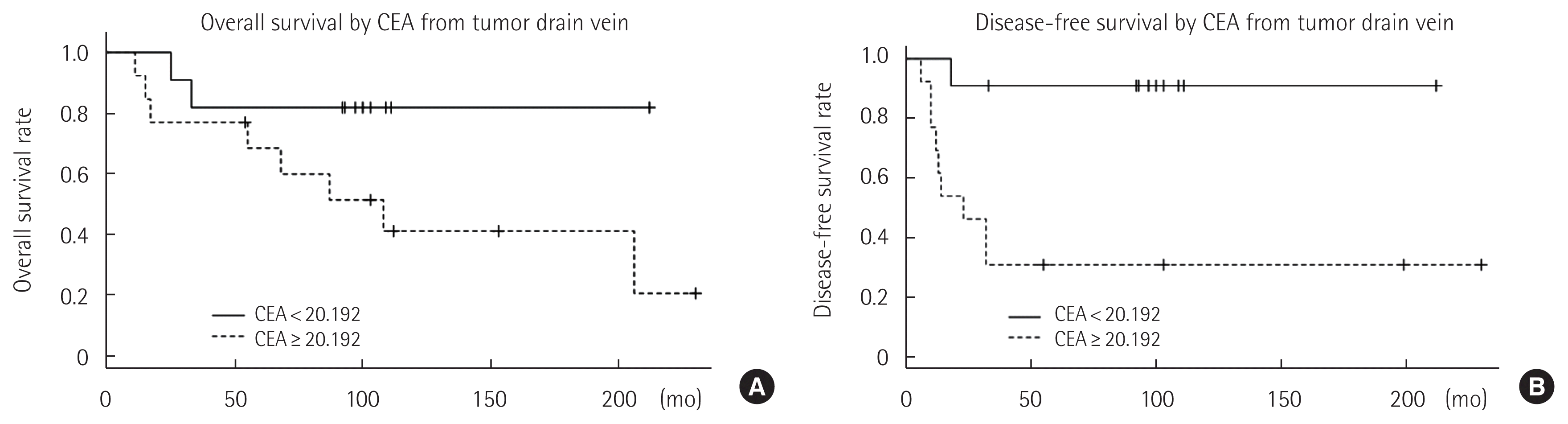
A subgroup analysis was performed to determine whether the dCEA level has prognostic significance in patients with a normal pCEA level. Of 51 patients in this study, 24 (47.06%) had a pCEA level in the normal range of < 5.9425 ng/mL; these patients were divided into 2 groups according to whether their dCEA level was below the calculated cut-off value (20.192 ng/mL). Kaplan-Meier survival analysis revealed that patients with a dCEA level = 20.192 ng/mL showed significantly poorer disease-free survival than those with dCEA < 20.192 ng/mL (P= 0.009) (Fig. 4). A Cox proportional hazard model revealed that none of the analyzed variables, including the dCEA level, were significant prognostic factors for both disease-free and overall survival.
DISCUSSION
CEA is a tumor marker generally used as an early indicator of tumor recurrence and prognosis for CRC [10,11]. Consistent elevation of serum CEA levels is a concerning sign of disease recurrence, and correlates sufficiently with CRC activity. For that reason, routine measurement of preoperative CEA levels before colorectal surgery for CRC patients is primarily suggested for subsequent postoperative surveillance by the Guidelines of the National Comprehensive Cancer Network (NCCN) and the European Society of Medical Oncology (ESMO) [1,12,13].
However, the significance of preoperative CEA levels as a prognostic factor for recurrence has clear limits [14–16]. Serum CEA has relatively low sensitivity, which may be attributed in part to non-CEA producing tumors that produce false negative results [3]. It is also well known that low-level elevation of CEA, between 5 and 10 ng/mL, has a substantial likelihood of representing false-positive elevation; Litvak et al. found that the 93% of isolated, single-occurrence elevations of CEA level with spontaneous normalization were low-level elevations of between 5.1 and 10.0 ng/mL [7].
The pCEA level is influenced by various pathophysiologic conditions including production of cancer cells [17], excretion of CEA into surrounding tissues, and entry into the lymphatic system and blood stream [18,19]. In that sense, measuring dCEA is considered to be more likely to reflect the status of the tumor [17,19]. The ability of the level of dCEA to predict CRC prognosis has not yet been established and is not clinically used. Therefore, in this study, we have attempted to evaluate the possibility of dCEA levels as prognostic markers in CRC compared with pCEA levels.
There was no significant difference in the overall survival rate and disease-free survival rate between patients with normal pCEA and increased pCEA values. However, both the overall survival rate (P= 0.033) and the disease-free survival rate (P= 0.009) of the group with normal dCEA levels were significantly better than those in the group with increased dCEA levels. Elevated dCEA levels were a significant prognostic factor for overall survival and disease-free survival in the Cox proportional hazard model analysis (HR= 399; 95% CI, 16.4–9,747; P< 0.001; HR= 9.39; 95% CI, 1.29–68.006; P = 0.026). These results suggest that dCEA levels may be used to predict prognosis or survival in patients with CRC more accurately than pCEA. The results concur with a previous study of Tabuchi et al. [20] that measurement of dCEA levels is more effective in patients with a poor prognosis than measurement of pCEA levels.
Interestingly, when dCEA was compared in patients with normal pCEA, overall survival rates of patients with normal dCEA was better and disease-free survival rates (P= 0.003) was significantly better than those with elevated dCEA. This result suggests that dCEA levels are more closely related to antigen levels released from cancer cells than pCEA levels, for two reasons. First, CEA is drained from cancer lesions primarily by the hematogenous portal system via the DV, but not by the thoracic duct of the lymphatic system [19]. Second, CEA levels in the PV may be influenced by degradation in the liver [21] or reabsorption in the intestinal wall [22].
Although dCEA may be more useful than pCEA for prediction of the prognosis of CRC, blood collection from a DV is a highly invasive procedure and follow-up collection after surgery is difficult. As serial collection of dCEA is difficult, both dCEA and pCEA are required to predict the prognosis of CRC. There are also concerns about complications of blood collection from a tumor DV. However, during surgery, draining venous blood can be collected without complications, and there were no postoperative complications associated with blood collection from tumor DVs.
Although this study is limited by its retrospective nature and small size, it demonstrated that the determination of dCEA levels can be useful as a predictive and prognostic marker in patients with CRC. The findings of this study increase the subsequent need for further investigations of the usefulness of dCEA as a tumor marker in various clinical settings such as CRC screening, surveillance after curative resection, and monitoring response to chemotherapy in patients with advanced disease.
While concerns exist about serial blood collection from a DV, the current study suggests that dCEA levels can be used as prognostic markers in patients with colon cancer more accurately than pCEA.